The impact of S- and G2-checkpoint response on the fidelity of G1-arrest by cisplatin and its comparison to a non-cross-resistant platinum(IV) analog
- PMID: 21592546
- PMCID: PMC3601787
- DOI: 10.1016/j.ygyno.2011.04.034
The impact of S- and G2-checkpoint response on the fidelity of G1-arrest by cisplatin and its comparison to a non-cross-resistant platinum(IV) analog
Abstract
Objective: Cisplatin is a DNA-damaging antitumor agent that is highly effective in treating ovarian cancer. It activates the p53/p21 pathway for its cytotoxic mode of action, but it does not induce p21-dependent cell cycle arrest in G1. Therefore, we investigated this paradox, and used the model analog DAP as a positive control for p21-dependent G1-arrest.
Methods: Studies were conducted in p53-proficient ovarian A2780 tumor cells to examine Cdk activity, cell cycle distribution and DNA damage signaling after cisplatin or DAP in combination with the mitotic inhibitor nocodazole.
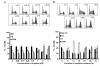
Results: Cisplatin consistently induced transient S-phase arrest by inhibiting Cdk2/cyclin A complex in S-phase at 12 h and then a durable G2/M-arrest by inhibiting Cdc2/cyclin B complex at 12-18 h. These inhibitions were associated with Chk1 and Chk2 activation and resultant increase in inhibitory tyrosine phosphorylation of Cdk2 and Cdc2. Cisplatin also potently inhibited G1-phase Cdk4/cyclin D1 and Cdk2/cyclin E activities at ~18 h. In agreement, exposure of cisplatin-treated A2780, HCT-116(p53-/-) and HCT-116(p21-/-) tumor cells to nocodazole revealed limited G1-arrest that was dependent on p53 and p21. In contrast, the durable G1-arrest by DAP, which failed to activate Chk1 and Chk2, was unaffected by nocodazole.
Conclusions: Cisplatin induced G1-arrest, but at an attenuated level. This was primarily due to orchestration of Cdk inhibition in S-phase first, then in G2, and finally in G1 that effectively blocked cells in G2 and prevented cells from progressing and arresting in G1. These studies demonstrate that cisplatin unequivocally activates G1-checkpoint response, but the fidelity of G1-arrest is compromised by Chk1/2 activation and checkpoint response in S- and G2/M-phase.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


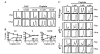


References
-
- Eastman A. Cell cycle checkpoints and their impact on anticancer therapeutic strategies. J Cell Biochem. 2004;91:223–31. - PubMed
-
- Samuel T, Weber HO, Funk JO. Linking DNA damage to cell cycle checkpoints. Cell Cycle. 2002;1:162–8. - PubMed
-
- Schwartz GK. Development of cell cycle active drugs for the treatment of gastrointestinal cancers: a new approach to cancer therapy. J Clin Oncol. 2005;23:4499–508. - PubMed
-
- Eastman A, Kohn EA, Brown MK, Rathman J, Livingstone M, Blank DH, Gribble GW. A novel indolocarbazole, ICP-1, abrogates DNA damage-induced cell cycle arrest and enhances cytotoxicity: similarities and differences to the cell cycle checkpoint abrogator UCN-01. Mol Cancer Ther. 2002;1:1067–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

