Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles
- PMID: 21592562
- PMCID: PMC3716294
- DOI: 10.1016/j.biomaterials.2011.04.046
Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles
Abstract
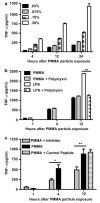
The inflammatory response to prosthetic implant-derived wear particles is the primary cause of bone loss and aseptic loosening of implants, but the mechanisms by which macrophages recognize and respond to particles remain unknown. Studies of innate immunity demonstrate that Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPS). All TLRs signal through myeloid differentiation factor 88 (MyD88), except TLR3 which signals through TIR domain containing adapter inducing interferon-beta (TRIF), and TLR4 which signals through both MyD88 and TRIF. We hypothesized that wear-debris particles may act as PAMPs/DAMPs and activate macrophages via TLRs. To test this hypothesis, we first demonstrated that inhibition of MyD88 decreases polymethylmethacrylate (PMMA) particle-induced production of TNF-α in RAW 264.7 macrophages. Next we compared particle-induced production of TNF-α among MyD88 knockout (MyD88(-/-)), TRIF knockout (TRIF(-/-)), and wild type (WT) murine macrophages. Relative to WT, disruption of MyD88 signaling diminished, and disruption of TRIF amplified the particle-induced production of TNF-α. Gene expression data indicated that this latter increase in TNF-α was due to a compensatory increase in expression of MyD88 associated components of the TLR pathway. Finally, using an in vivo model, MyD88(-/-) mice developed less particle-induced osteolysis than WT mice. These results indicate that the response to PMMA particles is partly dependent on MyD88, presumably as part of TLR signaling; MyD88 may represent a therapeutic target for prevention of wear debris-induced periprosthetic osteolysis.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



References
-
- Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001:66–70. - PubMed
-
- Day MJ, Butterworth SJ, Palmer MR, Case CP. Characterization of wear debris associated with aseptic loosening of a canine hip prosthesis. J Comp Pathol. 1998;119:89–93. - PubMed
-
- Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–86. - PubMed
-
- Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop Relat Res. 2001:71–7. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

