Antibody recognition of a human chorionic gonadotropin epitope (hCGbeta66-80) depends on local structure retained in the free peptide
- PMID: 21592960
- PMCID: PMC3137075
- DOI: 10.1074/jbc.M111.246637
Antibody recognition of a human chorionic gonadotropin epitope (hCGbeta66-80) depends on local structure retained in the free peptide
Abstract
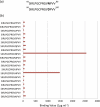
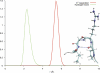
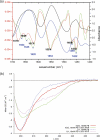
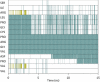
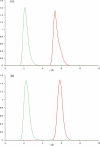
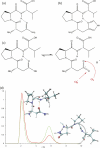
Human chorionic gonadotropin (hCG) is an important biomarker in pregnancy and oncology, where it is routinely detected and quantified by specific immunoassays. Intelligent epitope selection is essential to achieving the required assay performance. We present binding affinity measurements demonstrating that a typical β3-loop-specific monoclonal antibody (8G5) is highly selective in competitive immunoassays and distinguishes between hCGβ(66-80) and the closely related luteinizing hormone (LH) fragment LHβ(86-100), which differ only by a single amino acid residue. A combination of optical spectroscopic measurements and atomistic computer simulations on these free peptides reveals differences in turn type stabilized by specific hydrogen bonding motifs. We propose that these structural differences are the basis for the observed selectivity in the full protein.
Figures
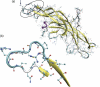

References
-
- Lapthorn A. J., Harris D. C., Littlejohn A., Lustbader J. W., Canfield R. E., Machin K. J., Morgan F. J., Isaacs N. W. (1994) Nature 369, 455–461 - PubMed
-
- Cole L. A. (2007) Placenta 28, 977–986 - PubMed
-
- O'Connor J. F., Birken S., Lustbader J. W., Krichevsky A., Chen Y., Canfield R. E. (1994) Endocr. Rev. 15, 650–683 - PubMed
-
- Sturgeon C. M., McAllister E. J. (1998) Ann. Clin. Biochem. 35, 460–491 - PubMed
-
- Gronowski A. M., Grenache D. G. (2009) Clin. Chem. 55, 1447–1449 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

