Fully human monoclonal antibody directed to proteolytic cleavage site in severe acute respiratory syndrome (SARS) coronavirus S protein neutralizes the virus in a rhesus macaque SARS model
- PMID: 21592986
- PMCID: PMC7107252
- DOI: 10.1093/infdis/jir084
Fully human monoclonal antibody directed to proteolytic cleavage site in severe acute respiratory syndrome (SARS) coronavirus S protein neutralizes the virus in a rhesus macaque SARS model
Abstract
Background: There is still no effective method to prevent or treat severe acute respiratory syndrome (SARS), which is caused by SARS coronavirus (CoV). In the present study, we evaluated the efficacy of a fully human monoclonal antibody capable of neutralizing SARS-CoV in vitro in a Rhesus macaque model of SARS.
Methods: The antibody 5H10 was obtained by vaccination of KM mice bearing human immunoglobulin genes with Escherichia coli-producing recombinant peptide containing the dominant epitope of the viral spike protein found in convalescent serum samples from patients with SARS.
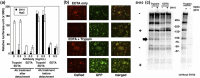
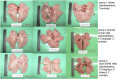
Results: 5H10, which recognized the same epitope that is also a cleavage site critical for the entry of SARS-CoV into host cells, inhibited propagation of the virus and pathological changes found in Rhesus macaques infected with the virus through the nasal route. In addition, we analyzed the mode of action of 5H10, and the results suggested that 5H10 inhibited fusion between the virus envelope and host cell membrane. 5H10 has potential for use in prevention and treatment of SARS if it reemerges.
Conclusions: This study represents a platform to produce fully human antibodies against emerging infectious diseases in a timely and safe manner.
Figures


References
-
- Guan Y, Zheng BJ, He YQ, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–8. - PubMed
-
- Marra MA, Jones SJ, Astell CR, et al. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–404. - PubMed
-
- Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

