Defense from the Group A Streptococcus by active and passive vaccination with the streptococcal hemoprotein receptor
- PMID: 21592989
- PMCID: PMC3096790
- DOI: 10.1093/infdis/jir149
Defense from the Group A Streptococcus by active and passive vaccination with the streptococcal hemoprotein receptor
Abstract
Background: The worldwide burden of the Group A Streptococcus (GAS) primary infection and sequelae is considerable, although immunization programs with broad coverage of the hyper variable GAS are still missing. We evaluate the streptococcal hemoprotein receptor (Shr), a conserved streptococcal protein, as a vaccine candidate against GAS infection.
Methods: Mice were immunized intraperitoneally with purified Shr or intranasally with Shr-expressing Lactococcus lactis. The resulting humoral response in serum and secretions was determined. We evaluated protection from GAS infection in mice after active or passive vaccination with Shr, and Shr antiserum was tested for bactericidal activity.
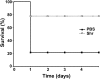
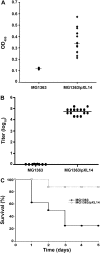
Results: A robust Shr-specific immunoglobulin (Ig) G response was observed in mouse serum after intraperitoneal vaccination with Shr. Intranasal immunization elicited both a strong IgG reaction in the serum and a specific IgA reaction in secretions. Shr immunization in both models allowed enhanced protection from systemic GAS challenge. Rabbit Shr antiserum was opsonizing, and mice that were administrated with Shr antiserum prior to the infection demonstrated a significantly higher survival rate than did mice treated with normal rabbit serum.
Conclusions: Shr is a promising vaccine candidate that is capable of eliciting bactericidal antibody response and conferring immunity against systemic GAS infection in both passive and active vaccination models.
Figures
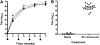
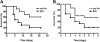
References
-
- Steer AC, Danchin MH, Carapetis JR. Group A streptococcal infections in children. J Paediatr Child Health. 2007;43:203–13. - PubMed
-
- Guilherme L, Fae K, Oshiro SE, Kalil J. Molecular pathogenesis of rheumatic fever and rheumatic heart disease. Expert Rev Mol Med. 2005;7:1–15. - PubMed
-
- Guilherme L, Ramasawmy R, Kalil J. Rheumatic fever and rheumatic heart disease: genetics and pathogenesis. Scand J Immunol. 2007;66:199–207. - PubMed
-
- Snider LA, Swedo SE. Post-streptococcal autoimmune disorders of the central nervous system. Curr Opin Neurol. 2003;16:359–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

