Randomized clinical trial of aspirin and simvastatin for pulmonary arterial hypertension: ASA-STAT
- PMID: 21593252
- PMCID: PMC3427737
- DOI: 10.1161/CIRCULATIONAHA.110.015693
Randomized clinical trial of aspirin and simvastatin for pulmonary arterial hypertension: ASA-STAT
Abstract
Background: Pulmonary arterial hypertension (PAH) is a progressive disease that causes exercise limitation, heart failure, and death. We aimed to determine the safety and efficacy of aspirin and simvastatin in PAH.
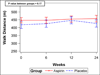
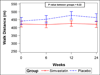
Methods and results: We performed a randomized, double-blind, placebo-controlled 2×2 factorial clinical trial of aspirin and simvastatin in patients with PAH receiving background therapy at 4 centers. A total of 92 patients with PAH were to be randomized to aspirin 81 mg or matching placebo and simvastatin 40 mg or matching placebo. The primary outcome was 6-minute walk distance at 6 months. Sixty-five subjects had been randomized when the trial was terminated by the Data Safety and Monitoring Board after an interim analysis showed futility in reaching the primary end point for simvastatin. After adjustment for baseline 6-minute walk distance, there was no significant difference in the 6-minute walk distance at 6 months between aspirin (n=32) and placebo (n=33; placebo-corrected difference −0.5 m, 95% confidence interval −28.4 to 27.4 m; P=0.97) or between simvastatin (n=32) and placebo (n=33; placebo-corrected difference −27.6 m, 95% confidence interval −59.6 to 4.3 m; P=0.09). There tended to be more major bleeding episodes with aspirin than with placebo (4 events versus 1 event, respectively; P=0.17).
Conclusions: Neither aspirin nor simvastatin had a significant effect on the 6-minute walk distance, although patients randomized to simvastatin tended to have a lower 6-minute walk distance at 6 months. These results do not support the routine treatment of patients with PAH with these medications.
Trial registration: ClinicalTrials.gov NCT00384865.
Figures





Comment in
-
Future of clinical trials for pulmonary hypertension.Circulation. 2011 Jun 28;123(25):2919-21. doi: 10.1161/CIRCULATIONAHA.111.037762. Circulation. 2011. PMID: 21709071 No abstract available.
References
-
- Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70–75. - PubMed
-
- Robbins IM, Kawut SM, Yung D, Reilly MP, Lloyd W, Cunningham G, Loscalzo J, Kimmel SE, Christman BW, Barst RJ. A study of aspirin and clopidogrel in idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;27:578–584. - PubMed
-
- Adatia I, Barrow SE, Stratton PD, Miall-Allen VM, Ritter JM, Haworth SG. Thromboxane A2 and prostacyclin biosynthesis in children and adolescents with pulmonary vascular disease. Circulation. 1993;88:2117–2122. - PubMed
-
- Barst RJ, Stalcup SA, Steeg CN, Hall JC, Frosolono MF, Cato AE, Mellins RB. Relation of arachidonate metabolites to abnormal control of the pulmonary circulation in a child. Am Rev Respir Dis. 1985;131:171–177. - PubMed
-
- Ichida F, Uese K, Hamamichi Y, Hashimoto I, Tsubata S, Fukahara K, Murakami A, Miyawaki T. Chronic effects of oral prostacyclin analogue on thromboxane A2 and prostacyclin metabolites in pulmonary hypertension. Acta Paediatr Jpn. 1998;40:14–19. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

