Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family
- PMID: 21593311
- PMCID: PMC3154647
- DOI: 10.1523/JNEUROSCI.6194-10.2011
Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family
Abstract
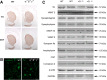
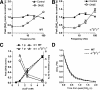
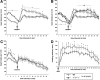
The synucleins (α, β, and γ) are highly homologous proteins thought to play a role in regulating neurotransmission and are found abundantly in presynaptic terminals. To overcome functional overlap between synuclein proteins and to understand their role in presynaptic signaling from mesostriatal dopaminergic neurons, we produced mice lacking all three members of the synuclein family. The effect on the mesostriatal system was assessed in adult (4- to 14-month-old) animals using a combination of behavioral, biochemical, histological, and electrochemical techniques. Adult triple-synuclein-null (TKO) mice displayed no overt phenotype and no change in the number of midbrain dopaminergic neurons. TKO mice were hyperactive in novel environments and exhibited elevated evoked release of dopamine in the striatum detected with fast-scan cyclic voltammetry. Elevated dopamine release was specific to the dorsal not ventral striatum and was accompanied by a decrease of dopamine tissue content. We confirmed a normal synaptic ultrastructure and a normal abundance of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) protein complexes in the dorsal striatum. Treatment of TKO animals with drugs affecting dopamine metabolism revealed normal rate of synthesis, enhanced turnover, and reduced presynaptic striatal dopamine stores. Our data uniquely reveal the importance of the synuclein proteins in regulating neurotransmitter release from specific populations of midbrain dopamine neurons through mechanisms that differ from those reported in other neurons. The finding that the complete loss of synucleins leads to changes in dopamine handling by presynaptic terminals specifically in those regions preferentially vulnerable in Parkinson's disease may ultimately inform on the selectivity of the disease process.
Figures
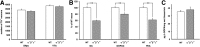



References
-
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
