Deletion of the hyperpolarization-activated cyclic nucleotide-gated channel auxiliary subunit TRIP8b impairs hippocampal Ih localization and function and promotes antidepressant behavior in mice
- PMID: 21593326
- PMCID: PMC3169171
- DOI: 10.1523/JNEUROSCI.0936-11.2011
Deletion of the hyperpolarization-activated cyclic nucleotide-gated channel auxiliary subunit TRIP8b impairs hippocampal Ih localization and function and promotes antidepressant behavior in mice
Abstract
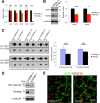
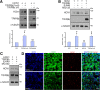
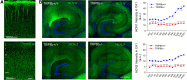
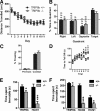
Output properties of neurons are greatly shaped by voltage-gated ion channels, whose biophysical properties and localization within axodendritic compartments serve to significantly transform the original input. The hyperpolarization-activated current, I(h), is mediated by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and plays a fundamental role in influencing neuronal excitability by regulating both membrane potential and input resistance. In neurons such as cortical and hippocampal pyramidal neurons, the subcellular localization of HCN channels plays a critical functional role, yet mechanisms controlling HCN channel trafficking are not fully understood. Because ion channel function and localization are often influenced by interacting proteins, we generated a knock-out mouse lacking the HCN channel auxiliary subunit, tetratricopeptide repeat-containing Rab8b-interacting protein (TRIP8b). Eliminating expression of TRIP8b dramatically reduced I(h) expression in hippocampal pyramidal neurons. Loss of I(h)-dependent membrane voltage properties was attributable to reduction of HCN channels on the neuronal surface, and there was a striking disruption of the normal expression pattern of HCN channels in pyramidal neuron dendrites. In heterologous cells and neurons, absence of TRIP8b increased HCN subunit targeting to and degradation by lysosomes. Mice lacking TRIP8b demonstrated motor learning deficits and enhanced resistance to multiple tasks of behavioral despair with high predictive validity for antidepressant efficacy. We observed similar resistance to behavioral despair in distinct mutant mice lacking HCN1 or HCN2. These data demonstrate that interaction with the auxiliary subunit TRIP8b is a major mechanism underlying proper expression of HCN channels and I(h) in vivo, and suggest that targeting I(h) may provide a novel approach to treatment of depression.
Figures







References
-
- Antonenkov VD, Van Veldhoven PP, Waelkens E, Mannaerts GP. Substrate specificities of 3-oxoacyl-CoA thiolase A and sterol carrier protein 2/3-oxoacyl-CoA thiolase purified from normal rat liver peroxisomes. Sterol carrier protein 2/3-oxoacyl-CoA thiolase is involved in the metabolism of 2-methyl-branched fatty acids and bile acid intermediates. J Biol Chem. 1997;272:26023–26031. - PubMed
-
- Baes M, Gressens P, Baumgart E, Carmeliet P, Casteels M, Fransen M, Evrard P, Fahimi D, Declercq PE, Collen D, van Veldhoven PP, Mannaerts GP. A mouse model for Zellweger syndrome. Nat Genet. 1997;17:49–57. - PubMed
-
- Barbuti A, DiFrancesco D. Control of cardiac rate by “funny” channels in health and disease. Ann N Y Acad Sci. 2008;1123:213–223. - PubMed
-
- Baruscotti M, Barbuti A, Bucchi A. The cardiac pacemaker current. J Mol Cell Cardiol. 2010;48:55–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F30 NS064757/NS/NINDS NIH HHS/United States
- K08 NS041956/NS/NINDS NIH HHS/United States
- NS059934/NS/NINDS NIH HHS/United States
- NS05595/NS/NINDS NIH HHS/United States
- R01 AG017139/AG/NIA NIH HHS/United States
- R56 AG017139/AG/NIA NIH HHS/United States
- RF1 AG017139/AG/NIA NIH HHS/United States
- R01 MH048432/MH/NIMH NIH HHS/United States
- R01 NS059934/NS/NINDS NIH HHS/United States
- AS2126/AS/Autism Speaks/United States
- R01 HD069560/HD/NICHD NIH HHS/United States
- K99 AG031574/AG/NIA NIH HHS/United States
- R00 AG031574/AG/NIA NIH HHS/United States
- MH048432/MH/NIMH NIH HHS/United States
- AG017139/AG/NIA NIH HHS/United States
- R01 MH081164/MH/NIMH NIH HHS/United States
- NS064757/NS/NINDS NIH HHS/United States
- MH081164/MH/NIMH NIH HHS/United States
- AG031574/AG/NIA NIH HHS/United States
- R21 HD065290/HD/NICHD NIH HHS/United States
- HD065290/HD/NICHD NIH HHS/United States
- R01 GM066181/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
