Involvement of adenosine A2A receptors in engulfment-dependent apoptotic cell suppression of inflammation
- PMID: 21593381
- PMCID: PMC3395167
- DOI: 10.4049/jimmunol.1002284
Involvement of adenosine A2A receptors in engulfment-dependent apoptotic cell suppression of inflammation
Abstract
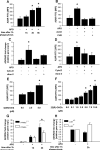
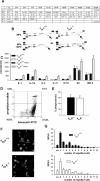
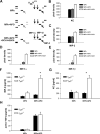
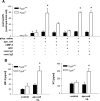
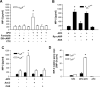
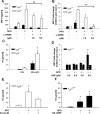
Efficient execution of apoptotic cell death followed by efficient clearance mediated by professional macrophages is a key mechanism in maintaining tissue homeostasis. Removal of apoptotic cells usually involves three central elements: 1) attraction of phagocytes via soluble "find me" signals, 2) recognition and phagocytosis via cell surface-presenting "eat me" signals, and 3) suppression or initiation of inflammatory responses depending on additional innate immune stimuli. Suppression of inflammation involves both direct inhibition of proinflammatory cytokine production and release of anti-inflammatory factors, which all contribute to the resolution of inflammation. In the current study, using wild-type and adenosine A(2A) receptor (A2AR) null mice, we investigated whether A2ARs, known to mediate anti-inflammatory signals in macrophages, participate in the apoptotic cell-mediated immunosuppression. We found that macrophages engulfing apoptotic cells release adenosine in sufficient amount to trigger A2ARs, and simultaneously increase the expression of A2ARs, as a result of possible activation of liver X receptor and peroxisome proliferators activated receptor δ. In macrophages engulfing apoptotic cells, stimulation of A2ARs suppresses the NO-dependent formation of neutrophil migration factors, such as macrophage inflammatory protein-2, using the adenylate cyclase/protein kinase A pathway. As a result, loss of A2ARs results in elevated chemoattractant secretion. This was evident as pronounced neutrophil migration upon exposure of macrophages to apoptotic cells in an in vivo peritonitis model. Altogether, our data indicate that adenosine is one of the soluble mediators released by macrophages that mediate engulfment-dependent apoptotic cell suppression of inflammation.
Figures

References
-
- Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol. Today. 1993;14:131–136. - PubMed
-
- Monoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, Herrmann M. SLE--a disease of clearance deficiency? Rheumatology. 2005;44:1101–1107. - PubMed
-
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

