Nicotinic excitation of serotonergic projections from dorsal raphe to the nucleus accumbens
- PMID: 21593391
- PMCID: PMC3154831
- DOI: 10.1152/jn.00575.2010
Nicotinic excitation of serotonergic projections from dorsal raphe to the nucleus accumbens
Abstract
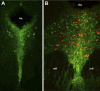
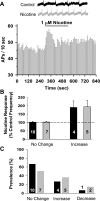
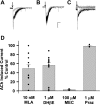
Tobacco use is a major public health problem, and although many smokers report that they want to quit, only a small percentage succeed. Side effects associated with nicotine withdrawal, including depression, anxiety, and restlessness, certainly contribute to the low success rate. The dorsal raphe nucleus (DRN) is a serotonergic center with many functions, including control of mood and emotional state. We investigated the effect of nicotine on DRN neurons that project to the nucleus accumbens (NAc), an area involved in reward-related behaviors. Using a retrograde labeling method, we found that 75% of DRN-NAc projection neurons are serotonergic. In coronal slices that include the DRN, whole cell recordings were conducted on neurons identified by fluorescent backlabeling from NAc or randomly selected within the nucleus. Nicotine increased action potential firing rates in a subset of DRN neurons. Voltage-clamp recording revealed nicotinic acetylcholine receptor (nAChR)-mediated inward currents that contribute to the nicotine-induced excitation. Nicotinic receptors also indirectly affect excitability by modulating synaptic inputs to these neurons. Nicotine enhanced excitatory glutamatergic inputs to a subset of DRN-NAc projection neurons, while inhibitory γ-aminobutyric acid (GABA)ergic inputs were modulated either positively or negatively in a subset of these neurons. The net effect of nAChR activation is enhancement of serotonergic output from DRN to the NAc, which may contribute to the effects of nicotine on mood and affect.
Figures



References
-
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. JAMA 264: 1541–1545, 1990 - PubMed
-
- Benloucif S, Keegan MJ, Galloway MP. Serotonin-facilitated dopamine release in vivo: pharmacological characterization. J Pharmacol Exp Ther 265: 373–377, 1993 - PubMed
-
- Bitner RS, Nikkel AL. Alpha-7 nicotinic receptor expression by two distinct cell types in the dorsal raphe nucleus and locus coeruleus of rat. Brain Res 938: 45–54, 2002 - PubMed
-
- Bitner RS, Nikkel AL, Curzon P, Donnelly-Roberts DL, Puttfarcken PS, Namovic M, Jacobs IC, Meyer MD, Decker MW. Reduced nicotinic receptor-mediated antinociception following in vivo antisense knock-down in rat. Brain Res 871: 66–74, 2000 - PubMed
-
- Centers for Disease Control and Prevention The Health Consequences of Smoking: a Report of the Surgeon General. Atlanta, GA: U. S. Department of Health and Human Services, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

