Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity
- PMID: 21593411
- PMCID: PMC3111285
- DOI: 10.1073/pnas.1106467108
Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity
Abstract
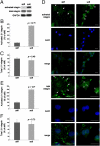
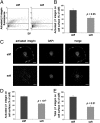
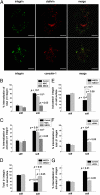
The mechanism by which ECM elasticity induces lineage specification of stem cells has not been clearly understood. Integrins are well-documented mechanosensors that are positioned at the beginning of the sensing pathway. By using an antibody specifically recognizing the active conformation of β1 integrin, we observed that β1 integrin activation in bone marrow mesenchymal stem cells (BMMSCs) was induced by soft substrate to a significantly greater degree than by stiff substrate. In contrast, however, the level of cell surface integrin on soft substrate was significantly lower than that on stiff substrate. Soft substrate markedly enhanced the internalization of integrin, and this internalization was mediated mainly through caveolae/raft-dependent endocytosis. The inhibition of integrin internalization blocked the neural lineage specification of BMMSCs on soft substrate. Furthermore, soft substrate also repressed the bone morphogenetic protein (BMP)/Smad pathway at least partially through integrin-regulated BMP receptor endocytosis. A theoretical analysis based on atomic force microscopy (AFM) data indicated that integrin-ligand complexes are more easily ruptured on soft substrate; this outcome may contribute to the enhancement of integrin internalization on soft substrate. Taken together, our results suggest that ECM elasticity affects integrin activity and trafficking to modulate integrin BMP receptor internalization, thus contributing to stem cell lineage specification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
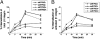

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

