Phosphorylation by MPK6: a conserved transcriptional modification mediates nitrate reductase activation and NO production?
- PMID: 21593598
- PMCID: PMC3218497
- DOI: 10.4161/psb.6.6.15308
Phosphorylation by MPK6: a conserved transcriptional modification mediates nitrate reductase activation and NO production?
Abstract
Nitrate reductase is a central enzyme of nitrogen assimilation in plants. In a recent work, we have revealed MPK6 could phosphorylate Arabidopsis NIA2 at the serine 627 in hinge 2 region, this phosporylation may represent a rapid activation mechnism when plant need excessive nitrate reduction. Interestingly, all eukaryotic NRs have conserved docking sequence in their FAD domains, and many plant NR proteins have the conserved MAPK phosphorylation site. Those results indicated the MAPK cascades, the conserved signaling pathway also involved in lateral root development, mediated of NR phosporylation and NO generation. We noticed that the phosphorylation of S627 residue by MPK6 have a specially influence on the NO generation activity of NIA2. Although no homology of mammalian NOS has been identified in plants, NR may still share a similar regulation mechanism with mammalian NOS.
Figures
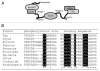
Comment on
- Wang P, Du Y, Li Y, Ren D, Song CP. Hydrogen peroxide-mediated activation of MAP Kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell. 2010:2981–2998. doi: 10.1105/tpc.109.0729. doi: 10.1105/tpc.109.0729
References
-
- Campbell WH. Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Ann Rev Plant Physiol Plant Molec Biol. 1999;50:277–303. - PubMed
-
- Lillo C, Lea US, Leydecker MT, Meyer C. Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in constitutive activation of the enzyme in vivo and nitrite accumulation. Plant J. 2003;35:566–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
