Is PrP the road to ruin?
- PMID: 21593729
- PMCID: PMC3098489
- DOI: 10.1038/emboj.2011.129
Is PrP the road to ruin?
Abstract
EMBO J 30 10, 2057–2070 (2011); published online March 25 2011
Neurodegenerative disorders are one among the most debilitating diseases of an ageing population. Understanding the mechanisms of neuronal cell death during pathogenesis of diseases such as Alzheimer's, Parkinson's, Huntington's, and prion diseases is key to addressing the options for treatment and prevention of brain deterioration. One feature of many such diseases is the accumulation of specific misfolded proteins. Often these misfolded proteins take the form of large amyloid fibrils or plaques, but recent observations implicate small soluble oligomers as the primary causes of neuronal dysfunction. How these misfolded proteins trigger cell death pathways is largely unknown, but some reports have suggested mediation by normal cellular prion protein (PrPC). In this issue, Resenberger et al (2011) provide evidence for membrane-anchored PrPC's role in recognizing a variety of β-sheet-rich protein conformers and transducing pro-apoptotic signals.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
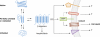
Comment on
-
The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication.EMBO J. 2011 May 18;30(10):2057-70. doi: 10.1038/emboj.2011.86. Epub 2011 Mar 25. EMBO J. 2011. PMID: 21441896 Free PMC article.
References
-
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G (2010) Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci USA 107: 2295–2300 - PMC - PubMed
-
- Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26: 267–298 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

