Heart regeneration
- PMID: 21593865
- PMCID: PMC4091722
- DOI: 10.1038/nature10147
Heart regeneration
Abstract
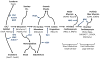
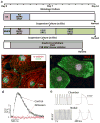
Heart failure plagues industrialized nations, killing more people than any other disease. It usually results from a deficiency of specialized cardiac muscle cells known as cardiomyocytes, and a robust therapy to regenerate lost myocardium could help millions of patients every year. Heart regeneration is well documented in amphibia and fish and in developing mammals. After birth, however, human heart regeneration becomes limited to very slow cardiomyocyte replacement. Several experimental strategies to remuscularize the injured heart using adult stem cells and pluripotent stem cells, cellular reprogramming and tissue engineering are in progress. Although many challenges remain, these interventions may eventually lead to better approaches to treat or prevent heart failure.
Figures

References
-
- Rumyantsev PP. In: Muscle Regeneration. Mauro A, editor. Raven Press; New York: 1979. pp. 335–355.
-
- Rumyantsev PP. Growth and hyperplasia of cardiac muscle cells. Harwood Academic Publishers; 1991.
-
- Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47(9):1777–1785. - PubMed
-
- Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

