RSK2 Binding Models Delineate Key Features for Activity
- PMID: 21593990
- PMCID: PMC3094916
RSK2 Binding Models Delineate Key Features for Activity
Abstract
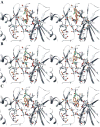
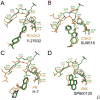
Due to its overexpression and activation in human cancer cells and tissues, an emerging molecular target in cancer therapeutics is p90 ribosomal s6 kinase 2 (RSK2). While a growing number of RSK2 inhibitors have been reported in the literature, only the crystal structure of RSK2 in complex with an AMP analogue provides a structural basis for understanding RSK2 inhibition. To remedy this, we used our fluorescence polarization assay to determine the RSK2 activity for a set of structurally diverse compounds, and followed this by modeling their binding modes in an all-atom, energy refined crystal structure of RSK2. These binding models reveal that Val131 and Leu147 are key interaction sites for potent RSK2 inhibition. This structure-based pharmacophore is an important tool for new lead discovery and refinement.
Figures
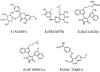


References
Grants and funding
LinkOut - more resources
Full Text Sources
