Proline-rich antimicrobial peptides: converging to a non-lytic mechanism of action
- PMID: 21594684
- PMCID: PMC11114787
- DOI: 10.1007/s00018-011-0721-7
Proline-rich antimicrobial peptides: converging to a non-lytic mechanism of action
Abstract
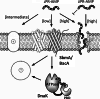
Proline-rich antimicrobial peptides are a group of cationic host defense peptides of vertebrates and invertebrates characterized by a high content of proline residues, often associated with arginine residues in repeated motifs. Those isolated from some mammalian and insect species, although not evolutionarily related, use a similar mechanism to selectively kill Gram-negative bacteria, with a low toxicity to animals. Unlike other types of antimicrobial peptides, their mode of action does not involve the lysis of bacterial membranes but entails penetration into susceptible cells, where they then act intracellularly. Some aspects of the transport system and cytoplasmic targets have been elucidated. These features make them attractive both as anti-infective lead compounds and as a new class of potential cell-penetrating peptides capable of internalising membrane-impermeant drugs into both bacterial and eukaryotic cells.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

