Analysis of CFTR interactome in the macromolecular complexes
- PMID: 21594790
- PMCID: PMC3178881
- DOI: 10.1007/978-1-61779-117-8_17
Analysis of CFTR interactome in the macromolecular complexes
Abstract
Cystic fibrosis transmembrane conductance regulator (CFTR) is a chloride channel localized primarily at the apical surface of epithelial cells lining the airway, gut, exocrine glands, etc., where it is responsible for transepithelial salt and water transport. A growing number of proteins have been reported to interact directly or indirectly with CFTR chloride channel, suggesting that CFTR might regulate the activities of other ion channels, receptors, and transporters, in addition to its role as a chloride conductor. Most interactions occur primarily between the opposing terminal tails (N or C) of CFTR and its binding partners, either directly or mediated through various PDZ domain-containing proteins. This chapter describes methods we developed to cross-link CFTR into a macromolecular complex to identify and analyze the assembly and regulation of CFTR-containing complexes in the plasma membrane.
Figures
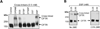

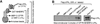
References
-
- Quinton PM. Chloride impermeability in cystic fibrosis. Nature. 1983;301:421–422. - PubMed
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. - PubMed
-
- Bear CE, Li CH, Kartner N, Bridges RJ, Jensen TJ, Ramjeesingh M, Riordan JR. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–818. - PubMed
-
- Welsh MJ, Tsui L-C, Boat TF, Beaudet AL. Cystic fibrosis. In: Scriver C, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Diseases: Membrane Transport Systems. New York, NY: McGraw-Hill; 1995. pp. 3799–3876.
-
- Li C, Naren AP. Macromolecular complexes of cystic fibrosis transmembrane conductance regulator and its interacting partners. Pharmacol. Ther. 2005;108:208–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

