In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP₂ receptor antagonist
- PMID: 21595651
- PMCID: PMC3246710
- DOI: 10.1111/j.1476-5381.2011.01495.x
In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP₂ receptor antagonist
Erratum in
- Br J Pharmacol. 2012 Jun;166(3):1192. Dosage error in article text
Abstract
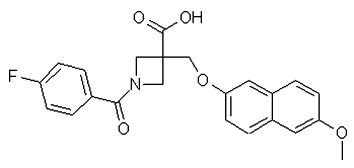
Background and purpose: Studies of the role of the prostaglandin EP(2) receptor) have been limited by the availability of potent and selective antagonist tools. Here we describe the in vitro/in vivo pharmacological characterization of a novel EP(2) receptor antagonist, PF-04418948 (1-(4-fluorobenzoyl)-3-{[(6-methoxy-2-naphthyl)oxy]methyl} azetidine-3-carboxylic acid).
Experimental approach: Functional antagonist potency was assessed in cell-based systems expressing human EP(2) receptors and native tissue preparations from human, dog and mouse. The selectivity of PF-04418948 was assessed against related receptors and a panel of GPCRs, ion channels and enzymes. The ability of PF-04418948 to pharmacologically block EP(2) receptor function in vivo was tested in rats.
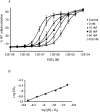
Key results: PF-04418948 inhibited prostaglandin E(2)(PGE(2))-induced increase in cAMP in cells expressing EP(2) receptors with a functional K(B) value of 1.8 nM. In human myometrium, PF-04418948 produced a parallel, rightward shift of the butaprost-induced inhibition of the contractions induced by electrical field stimulation with an apparent K(B) of 5.4 nM. In dog bronchiole and mouse trachea, PF-04418948 produced parallel rightward shifts of the PGE(2)-induced relaxation curve with a K(B) of 2.5 nM and an apparent K(B) of 1.3 nM respectively. Reversal of the PGE(2)-induced relaxation in the mouse trachea by PF-04418948 produced an IC(50) value of 2.7 nM. Given orally, PF-04418948 attenuated the butaprost-induced cutaneous blood flow response in rats. PF-04418948 was selective for EP(2) receptors over homologous and unrelated receptors, enzymes and channels.
Conclusions and implications: PF-04418948 is an orally active, potent and selective surmountable EP(2) receptor antagonist that should aid further elaboration of EP(2) receptor function.
© 2011 Pfizer Limited. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures




Comment in
-
At last, a truly selective EP₂ receptor antagonist.Br J Pharmacol. 2011 Dec;164(7):1845-6. doi: 10.1111/j.1476-5381.2011.01494.x. Br J Pharmacol. 2011. PMID: 21595650 Free PMC article.
References
-
- Abela AP, Daniel EE. Neural and myogenic effects of cyclooxygenase products on canine bronchial smooth muscle. Am J Physiol. 1995;268:L47–L55. Part 1. - PubMed
-
- Abramovitz M, Adam M, Boie Y, Carrière M, Denis D, Godbout C, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. - PubMed
-
- Bastien L, Sawyer N, Grygorczyk R, Metters KM, Adam M. Cloning, functional expression and characterization of the human prostaglandin E2 receptor EP2 subtype. J Biol Chem. 1994;269:11873–11877. - PubMed
-
- Birnbaum JE, Birkhead NC, Oronsky AL, Dessy F, Rihoux JP, VanHumbeeck L. Bronchodilator activity of a PGE2 analog in animals and in man. Prostaglandins. 1981;21:457–469. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

