Hypothalamic actions and interactions of alcohol and IGF-1 on the expression of glial receptor protein tyrosine phosphatase-β during female pubertal development
- PMID: 21595703
- PMCID: PMC3161137
- DOI: 10.1111/j.1530-0277.2011.01525.x
Hypothalamic actions and interactions of alcohol and IGF-1 on the expression of glial receptor protein tyrosine phosphatase-β during female pubertal development
Abstract
Background: Hypothalamic glial-neuronal communications are important for the activation of luteinizing hormone releasing hormone (LHRH) secretion at the time of puberty. As we have shown that alcohol (ALC) diminishes prepubertal LHRH secretion and delays puberty, we first assessed the effects of short-term ALC administration on the basal expression of a specific gene family involved in glial-neuronal communications. Second, as insulin-like growth factor-1 (IGF-1) is a critical regulator of LHRH secretion and the pubertal process, we then assessed whether IGF-1 could induce the expression of these signaling genes and determine whether ALC can block this affect.
Methods: Immature female rats were fed a liquid diet containing ALC for 6 days beginning when 27 days old. Control animals received either the companion isocaloric liquid diet or rat chow and water. Animals were decapitated on day 33, in the late juvenile stage of development. Medial basal hypothalamic (MBH) tissues were obtained for gene and protein analyses of glial receptor protein tyrosine phosphatase-β (RPTPβ) and the 2 neuronal components, contactin and contactin-associated protein 1 (Caspr1). In the second experiment, IGF-1 was administered into the third ventricle (3V) and the MBH removed 6 hours after peptide delivery, and the above-mentioned 3 genes were analyzed by real-time PCR. To determine whether this action was affected by ALC, immature female rats were administered either ALC (3 g/kg) or water via gastric gavage at 0900 hours. At 1030 hours, the ALC and control groups were subdivided such that half of the animals were injected into the 3V with IGF-1 and the other half with an equal volume of saline. Rats were killed 6 hours after the IGF-1 injection and MBHs collected.
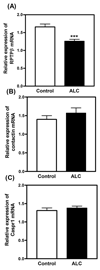
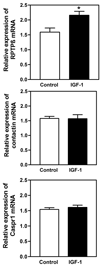
Results: Real-time PCR showed that when compared with control animals, ALC caused a marked decrease (p < 0.001) in the basal expression of the RPTPβ gene, but did not affect the expression of either contactin or Caspr1. Likewise, analysis by Western blotting demonstrated that ALC caused suppressed (p < 0.001) levels of the RPTPβ protein, with the expressions of both contactin and Caspr1 proteins being unaltered. In the second experiment, results showed that only the RPTPβ gene was stimulated (p < 0.05) by IGF-1 in the MBH 6 hours after peptide delivery. Assessments revealed that the IGF-1 induced increase (p < 0.01) in the expression of the RPTPβ gene was blocked by the presence of ALC.
Conclusions: Prepubertal ALC exposure is capable of interfering with hypothalamic glial-neuronal communications by suppressing the synthesis of the glial product, RPTPβ, which is required for binding to the contactin-Caspr1 complex on LHRH neuronal terminals, thus suggesting that this action of ALC contributes to its detrimental effects on the pubertal process.
Copyright © 2011 by the Research Society on Alcoholism.
Figures


References
-
- Anderson RA, Willis BR, Oswald C, Gupta A, Zaneveld L. Delayed male sexual maturation induced by chronic ethanol ingestion. Fed Proc. 1981;40:825–829.
-
- Bo WJ, Krueger WA, Rudeen PK, Symees SK. Ethanol-induced alterations in the morphology and function of the rat ovary. Anat Rec. 1982;202:255–260. - PubMed
-
- Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role of IGF-1. Endocrinology. 1999;140:2637–2640. - PubMed
-
- Dees WL, Dissen GA, Hiney JK, Lara F, Ojeda SR. Alcohol ingestion inhibits the increased secretion of puberty-related hormones in the developing female rhesus monkey. Endocrinology. 2000;141:1325–1331. - PubMed
-
- Dees WL, Hiney JK, Nyberg CL. Effects of ethanol on the reproductive neuroendocrine axis of prepubertal and adult female rats. Chapter 14. In: Sarkar DK, Barnes CD, editors. The Reproductive Neuroendocrinology of Aging and Drug Abuse. Boca Raton, FL: CRC Press; 1995. pp. 301–334.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

