Some lumbar sympathetic neurons develop a glutamatergic phenotype after peripheral axotomy with a note on VGLUT₂-positive perineuronal baskets
- PMID: 21596036
- PMCID: PMC3114170
- DOI: 10.1016/j.expneurol.2011.05.004
Some lumbar sympathetic neurons develop a glutamatergic phenotype after peripheral axotomy with a note on VGLUT₂-positive perineuronal baskets
Abstract
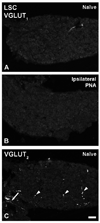
Glutamate is the main excitatory neurotransmitter in the nervous system, including in primary afferent neurons. However, to date a glutamatergic phenotype of autonomic neurons has not been described. Therefore, we explored the expression of vesicular glutamate transporter (VGLUT) types 1, 2 and 3 in lumbar sympathetic chain (LSC) and major pelvic ganglion (MPG) of naïve BALB/C mice, as well as after pelvic nerve axotomy (PNA), using immunohistochemistry and in situ hybridization. Colocalization with activating transcription factor-3 (ATF-3), tyrosine hydroxylase (TH), vesicular acetylcholine transporter (VAChT) and calcitonin gene-related peptide was also examined. Sham-PNA, sciatic nerve axotomy (SNA) or naïve mice were included. In naïve mice, VGLUT(2)-like immunoreactivity (LI) was only detected in fibers and varicosities in LSC and MPG; no ATF-3-immunoreactive (IR) neurons were visible. In contrast, PNA induced upregulation of VGLUT(2) protein and transcript, as well as of ATF-3-LI in subpopulations of LSC neurons. Interestingly, VGLUT(2)-IR LSC neurons coexpressed ATF-3, and often lacked the noradrenergic marker TH. SNA only increased VGLUT(2) protein and transcript in scattered LSC neurons. Neither PNA nor SNA upregulated VGLUT(2) in MPG neurons. We also found perineuronal baskets immunoreactive either for VGLUT(2) or the acetylcholinergic marker VAChT in non-PNA MPGs, usually around TH-IR neurons. VGLUT(1)-LI was restricted to some varicosities in MPGs, was absent in LSCs, and remained largely unaffected by PNA or SNA. This was confirmed by the lack of expression of VGLUT(1) or VGLUT(3) mRNAs in LSCs, even after PNA or SNA. Taken together, axotomy of visceral and non-visceral nerves results in a glutamatergic phenotype of some LSC neurons. In addition, we show previously non-described MPG perineuronal glutamatergic baskets.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures









References
-
- Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J. Histochem. Cytochem. 1992;40:1457–1463. - PubMed
-
- Aïoun J, Rampin O. Anatomical evidence for glutamatergic transmission in primary sensory neurons and onto postganglionic neurons controlling penile erection in rats: an ultrastructural study with neuronal tracing and immunocytochemistry. Cell. Tissue Res. 2006;323:359–375. - PubMed
-
- Apostolova G, Dechant G. Development of neurotransmitter phenotypes in sympathetic neurons. Auton. Neurosci. 2009;151:30–38. - PubMed
-
- Balazs R. Trophic effect of glutamate. Curr. Top. Med. Chem. 2006;6:961–968. - PubMed
-
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

