MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells
- PMID: 21596042
- PMCID: PMC3129445
- DOI: 10.1053/j.gastro.2011.04.010
MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells
Abstract
Background & aims: Gut-associated dendritic cells (DC) metabolize vitamin A into all-trans retinoic acid (RA), which is required to induce lymphocytes to localize to the gastrointestinal tract and promotes the differentiation of Foxp3+ regulatory T cells and IgA antibody-secreting cells. We investigated whether RA functions in a positive-feedback loop in DC to induce its own synthesis.
Methods: We measured levels of retinoids in intestinal tissues from mice and assessed the role of RA in the functional specialization of gut-associated DC in cell cultures and mice. We used pharmacologic antagonists to determine the signaling pathways involved in regulation of DC and used MyD88-/- mice to determine the contribution of Toll-like receptor signaling in RA-mediated effects on DC.
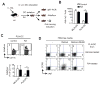
Results: The concentration of retinoids decreased in a proximal-to-distal gradient along the intestine, which correlated with the activity of gut-specific DC. Importantly, RA regulated the ability of gut-associated DC to produce RA, induce T cells to localize to the gastrointestinal tract, and generate regulatory T cells and IgA-secreting cells. RA was sufficient to induce its own production by extraintestinal DC in vitro and in vivo. RA-mediated regulation of DC required signaling through the mitogen-activated protein kinase signaling pathway and unexpectedly required MyD88, which is conventionally associated with Toll-like receptor, interleukin-1, and interleukin-18 signaling.
Conclusions: RA is necessary and sufficient to induce DC to regulate T-cell localization to the gastrointestinal tract and IgA secretion. Our findings also indicate crosstalk between the RA receptor and MyD88-dependent Toll-like receptor signaling pathways.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





References
-
- Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis. 2008;14:275–89. - PubMed
-
- Eksteen B, Adams DH. GSK-1605786, a selective small-molecule antagonist of the CCR9 chemokine receptor for the treatment of Crohn’s disease. IDrugs. 2010;13:472–781. - PubMed
-
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. - PubMed
-
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

