Mutations in ZBTB24 are associated with immunodeficiency, centromeric instability, and facial anomalies syndrome type 2
- PMID: 21596365
- PMCID: PMC3113345
- DOI: 10.1016/j.ajhg.2011.04.018
Mutations in ZBTB24 are associated with immunodeficiency, centromeric instability, and facial anomalies syndrome type 2
Abstract
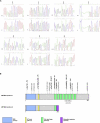
Autosomal-recessive immunodeficiency, centromeric instability, and facial anomalies (ICF) syndrome is mainly characterized by recurrent, often fatal, respiratory and gastrointestinal infections. About 50% of patients carry mutations in the DNA methyltransferase 3B gene (DNMT3B) (ICF1). The remaining patients carry unknown genetic defects (ICF2) but share with ICF1 patients the same immunological and epigenetic features, including hypomethylation of juxtacentromeric repeat sequences. We performed homozygosity mapping in five unrelated ICF2 patients with consanguineous parents and then performed whole-exome sequencing in one of these patients and Sanger sequencing in all to identify mutations in the zinc-finger- and BTB (bric-a-bric, tramtrack, broad complex)-domain-containing 24 (ZBTB24) gene in four consanguineously descended ICF2 patients. Additionally, we found ZBTB24 mutations in an affected sibling pair and in one patient for whom it was not known whether his parents were consanguineous. ZBTB24 belongs to a large family of transcriptional repressors that include members, such as BCL6 and PATZ1, with prominent regulatory roles in hematopoietic development and malignancy. These data thus indicate that ZBTB24 is involved in DNA methylation of juxtacentromeric DNA and in B cell development and/or B and T cell interactions. Because ZBTB24 is a putative DNA-binding protein highly expressed in the lymphoid lineage, we predict that by studying the molecular function of ZBTB24, we will improve our understanding of the molecular pathophysiology of ICF syndrome and of lymphocyte biology in general.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
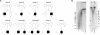

References
-
- Hagleitner M.M., Lankester A., Maraschio P., Hultén M., Fryns J.P., Schuetz C., Gimelli G., Davies E.G., Gennery A., Belohradsky B.H. Clinical spectrum of immunodeficiency, centromeric instability and facial dysmorphism (ICF syndrome) J. Med. Genet. 2008;45:93–99. - PubMed
-
- Hansen R.S. X inactivation-specific methylation of LINE-1 elements by DNMT3B: Implications for the Lyon repeat hypothesis. Hum. Mol. Genet. 2003;12:2559–2567. - PubMed
-
- Xu G.L., Bestor T.H., Bourc'his D., Hsieh C.L., Tommerup N., Bugge M., Hulten M., Qu X., Russo J.J., Viegas-Péquignot E. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402:187–191. - PubMed
-
- Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

