Rifampin, but not rifabutin, may produce opiate withdrawal in buprenorphine-maintained patients
- PMID: 21596492
- PMCID: PMC3272858
- DOI: 10.1016/j.drugalcdep.2011.04.013
Rifampin, but not rifabutin, may produce opiate withdrawal in buprenorphine-maintained patients
Abstract
Background: This series of studies examines the pharmacokinetic/pharmacodynamic interactions between buprenorphine, an opioid partial agonist increasingly used in treatment of opioid dependence, and rifampin, a medication used as a first line treatment for tuberculosis; or rifabutin, an alternative antituberculosis medication.
Methods: Opioid-dependent individuals on stable doses of buprenorphine/naloxone underwent two, 24-h blood sampling studies: (1) for buprenorphine pharmacokinetics and (2) following 15 days of rifampin 600 mg daily or rifabutin 300 mg daily for buprenorphine and rifampin or rifabutin pharmacokinetics.
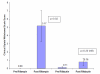
Results: Rifampin administration produced significant reduction in plasma buprenorphine concentrations (70% reduction in mean area under the curve (AUC); p=<0.001) and onset of opiate withdrawal symptoms in 50% of participants (p=0.02). While rifabutin administration to buprenorphine-maintained subjects resulted in a significant decrease in buprenorphine plasma concentrations (35% decrease in AUC; p<0.001) no opiate withdrawal was seen. Compared with historical control data, buprenorphine had no significant effect on rifampin pharmacokinetics, but was associated with 22% lower rifabutin mean AUC (p=0.009), although rifabutin and its active metabolite concentrations remained in the therapeutic range.
Conclusions: Rifampin is a more potent inducer of buprenorphine metabolism than rifabutin with pharmacokinetic and pharmacodynamic adverse consequences. Those patients requiring rifampin treatment for tuberculosis and receiving buprenorphine therapy are likely to require an increase in buprenorphine dose to prevent withdrawal symptoms. Rifabutin administration was associated with decreases in buprenorphine plasma concentrations, but no clinically significant adverse events were observed.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures



References
-
- Benedetti MS. Inducing properties of rifabutin, and effects on the pharmacokinetics and metabolism of concomitant drugs. Pharmacol. Res. 1995;32:177–187. - PubMed
-
- Brogden RN, Fitton A. Rifabutin: a review of its antimicrobial activity, pharmacokinentic properties and therapeutic efficacy. Drugs. 1994;47:983–1009. - PubMed
-
- Brown LS, Sawyer RC, Li R, Cobb MN, Colborn DC, Narang PK. Lack of a pharmacologic interaction between rifabutin and methadone in HIV-infected former injecting drug users. Drug Alcohol Depend. 1996;43:71–77. - PubMed
-
- Burman W, Gallican K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 2001;40:327–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

