Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)--associated tuberculous meningitis
- PMID: 21596680
- PMCID: PMC4340579
- DOI: 10.1093/cid/cir230
Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)--associated tuberculous meningitis
Abstract
Background: The optimal time to initiate antiretroviral therapy (ART) in human immunodeficiency virus (HIV)-associated tuberculous meningitis is unknown.
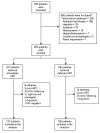
Methods: We conducted a randomized, double-blind, placebo-controlled trial of immediate versus deferred ART in patients with HIV-associated tuberculous meningitis to determine whether immediate ART reduced the risk of death. Antiretroviral drugs (zidovudine, lamivudine, and efavirenz) were started either at study entry or 2 months after randomization. All patients were treated with standard antituberculosis treatment, adjunctive dexamethasone, and prophylactic co-trimoxazole and were followed up for 12 months. We conducted intention-to-treat, per-protocol, and prespecified subgroup analyses.
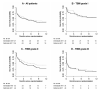
Results: A total of 253 patients were randomized, 127 in the immediate ART group and 126 in the deferred ART group; 76 and 70 patients died within 9 months in the immediate and deferred ART groups, respectively. Immediate ART was not significantly associated with 9-month mortality (hazard ratio [HR], 1.12; 95% confidence interval [CI], .81-1.55; P = .50) or the time to new AIDS events or death (HR, 1.16; 95% CI, .87-1.55; P = .31). The percentage of patients with severe (grade 3 or 4) adverse events was high in both arms (90% in the immediate ART group and 89% in the deferred ART group; P = .84), but there were significantly more grade 4 adverse events in the immediate ART arm (102 in the immediate ART group vs 87 in the deferred ART group; P = .04).
Conclusions: Immediate ART initiation does not improve outcome in patients presenting with HIV-associated tuberculous meningitis. There were significantly more grade 4 adverse events in the immediate ART arm, supporting delayed initiation of ART in HIV-associated tuberculous meningitis. Clinical Trials Registration. ISRCTN63659091.
Figures
Comment in
-
Poor prognosis of HIV-associated tuberculous meningitis regardless of the timing of antiretroviral therapy.Clin Infect Dis. 2011 Jun;52(11):1384-7. doi: 10.1093/cid/cir239. Clin Infect Dis. 2011. PMID: 21596681 Free PMC article. No abstract available.
-
[When to start antiretroviral therapy in patients with meningeal tuberculosis associated with human immunodeficiency virus?].Rev Clin Esp. 2012 Jan;212(1):43. doi: 10.1016/j.rce.2011.07.003. Rev Clin Esp. 2012. PMID: 22389915 Spanish. No abstract available.
References
-
- British HIV Association [Accessed 1 April 2011];BHIVA treatment guidelines for TB/HIV infection. 2005 http://www.bhiva.org/TBHIVCo_infection2005.aspx.
-
- Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. - PubMed
-
- World Health Organization [Accessed 1 April 2011];Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach—2010 revision. 2010 http://www.who.int/hiv/pub/arv/adult2010/en/index.html. - PubMed
-
- Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–51. - PubMed
-
- Berenguer J, Moreno S, Laguna F, et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med. 1992;326:668–72. - PubMed

