Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene
- PMID: 21596819
- PMCID: PMC3129369
- DOI: 10.1158/0008-5472.CAN-10-4041
Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene
Abstract
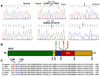
Thyroid cancer is the most common endocrine cancer, and targeted approaches to treat it pose considerable interest. In this study, we report the discovery of ALK gene mutations in thyroid cancer that may rationalize clinical evaluation of anaplastic lymphoma kinase (ALK) inhibitors in this setting. In undifferentiated anaplastic thyroid cancer (ATC), we identified two novel point mutations, C3592T and G3602A, in exon 23 of the ALK gene, with a prevalence of 11.11%, but found no mutations in the matched normal tissues or in well-differentiated thyroid cancers. These two mutations, resulting in L1198F and G1201E amino acid changes, respectively, both reside within the ALK tyrosine kinase domain where they dramatically increased tyrosine kinase activities. Similarly, these mutations heightened the ability of ALK to activate the phosphatidylinositol 3-kinase (PI3K)/Akt and mitogen-activated protein (MAP) kinase pathways in established mouse cells. Further investigations showed that these two ALK mutants strongly promoted cell focus formation, anchorage-independent growth, and cell invasion. Similar oncogenic properties were observed in the neuroblastoma-associated ALK mutants K1062M and F1174L but not in wild-type ALK. Overall, our results reveal two novel gain-of-function mutations of ALK in certain ATCs, and they suggest efforts to clinically evaluate the use of ALK kinase inhibitors to treat patients who harbor ATCs with these mutations.
©2011 AACR.
Figures
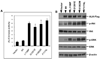


References
-
- Kelleher FC, McDermott R. The emerging pathogenic and therapeutic importance of the anaplastic lymphoma kinase gene. Eur J Cancer. 2010;46:2357–2368. - PubMed
-
- Ardini E, Magnaghi P, Orsini P, Galvani A, Menichincheri M. Anaplastic Lymphoma Kinase: Role in specific tumours and development of small molecule inhibitors for cancer therapy. Cancer Lett. 2010;299:81–94. - PubMed
-
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. - PubMed
-
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. - PubMed
-
- Ma Z, Hill DA, Collins MH, Morris SW, Sumegi J, Zhou M, et al. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37:98–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

