A murine model for catheter-associated candiduria
- PMID: 21596904
- PMCID: PMC3347865
- DOI: 10.1099/jmm.0.026294-0
A murine model for catheter-associated candiduria
Abstract
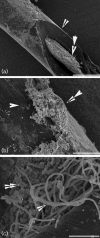
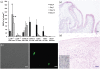
Candiduria is a common finding in hospitalized patients with indwelling urine-draining devices. Animal models for candiduria are not well-developed and, despite its prevalence and associated mortality, candiduria is understudied. The presence of Candida in urine does not imply disease because it is also a commensal. Biofilm formation on catheters and the host-pathogen interaction are likely to be important factors that contribute to the pathogenesis. The objective of this study was to establish a candiduria model in mice with indwelling catheters. Our data demonstrate that biofilm formation on indwelling catheters and persistent candiduria can be established in mice. The study supports the concept that biofilm formation contributes to persistence. It also outlines differences between catheter-related candiduria in mice and humans. Specifically, mice exhibit higher levels of leukocyturia. In addition, mean daily fungal burden in urine in the murine model is 10- to 100-fold lower than that in humans. These important findings must be taken into consideration when using this model to study host-pathogen interaction in the setting of candiduria.
Figures


Similar articles
-
Murine model for the evaluation of candiduria caused by Candida tropicalis from biofilm.Microb Pathog. 2018 Apr;117:170-174. doi: 10.1016/j.micpath.2018.02.036. Epub 2018 Feb 19. Microb Pathog. 2018. PMID: 29471135
-
Catheter-associated candiduria: Risk factors, medical interventions, and antifungal susceptibility.Am J Infect Control. 2015 Jul 1;43(7):e19-22. doi: 10.1016/j.ajic.2015.03.013. Epub 2015 Apr 24. Am J Infect Control. 2015. PMID: 25920705
-
Long-term follow-up of patients with candiduria.Eur J Clin Microbiol Infect Dis. 2011 Feb;30(2):137-40. doi: 10.1007/s10096-010-1061-5. Epub 2010 Sep 22. Eur J Clin Microbiol Infect Dis. 2011. PMID: 20857164
-
Candiduria: a review of clinical significance and management.Saudi J Kidney Dis Transpl. 2008 May;19(3):350-60. Saudi J Kidney Dis Transpl. 2008. PMID: 18445893 Review.
-
Candiduria: Evidence-based approach to management, are we there yet?J Mycol Med. 2017 Sep;27(3):293-302. doi: 10.1016/j.mycmed.2017.04.005. Epub 2017 May 10. J Mycol Med. 2017. PMID: 28501465 Review.
Cited by
-
Candida biofilms and the host: models and new concepts for eradication.Int J Microbiol. 2012;2012:845352. doi: 10.1155/2012/845352. Epub 2011 Nov 14. Int J Microbiol. 2012. PMID: 22164167 Free PMC article.
-
Candida albicans biofilm development on medically-relevant foreign bodies in a mouse subcutaneous model followed by bioluminescence imaging.J Vis Exp. 2015 Jan 27;(95):52239. doi: 10.3791/52239. J Vis Exp. 2015. PMID: 25651138 Free PMC article.
-
Candida albicans Biofilms and Human Disease.Annu Rev Microbiol. 2015;69:71-92. doi: 10.1146/annurev-micro-091014-104330. Annu Rev Microbiol. 2015. PMID: 26488273 Free PMC article. Review.
-
Development and regulation of single- and multi-species Candida albicans biofilms.Nat Rev Microbiol. 2018 Jan;16(1):19-31. doi: 10.1038/nrmicro.2017.107. Epub 2017 Oct 3. Nat Rev Microbiol. 2018. PMID: 29062072 Free PMC article. Review.
-
How Biofilm Growth Affects Candida-Host Interactions.Front Microbiol. 2020 Jun 25;11:1437. doi: 10.3389/fmicb.2020.01437. eCollection 2020. Front Microbiol. 2020. PMID: 32670252 Free PMC article. Review.
References
-
- Bartkowski D. P., Lanesky J. R. (1988). Emphysematous prostatitis and cystitis secondary to Candida albicans. J Urol 139, 1063–1065 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

