Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3-Bim axis
- PMID: 21597002
- PMCID: PMC3157683
- DOI: 10.1096/fj.11-183798
Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3-Bim axis
Abstract
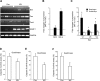
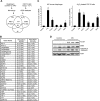
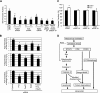
Mechanical ventilation (MV) is a life-saving measure in many critically ill patients. However, prolonged MV results in diaphragm dysfunction that contributes to the frequent difficulty in weaning patients from the ventilator. The molecular mechanisms underlying ventilator-induced diaphragm dysfunction (VIDD) remain poorly understood. We report here that MV induces myonuclear DNA fragmentation (3-fold increase; P<0.01) and selective activation of caspase 9 (P<0.05) and Bcl2-interacting mediator of cell death (Bim; 2- to 7-fold increase; P<0.05) in human diaphragm. MV also statistically significantly down-regulates mitochondrial gene expression and induces oxidative stress. In cultured muscle cells, we show that oxidative stress activates each of the catabolic pathways thought to underlie VIDD: apoptotic (P<0.05), proteasomal (P<0.05), and autophagic (P<0.01). Further, silencing Bim expression blocks (P<0.05) oxidative stress-induced apoptosis. Overlapping the gene expression profiles of MV human diaphragm and H₂O₂-treated muscle cells, we identify Fos, FoxO1, and Stat3 as regulators of Bim expression as well as of expression of the catabolic markers atrogin and LC3. We thus identify a novel Fos/FoxO1/Stat3-Bim intrinsic apoptotic pathway and establish the centrality of oxidative stress in the development of VIDD. This information may help in the design of specific drugs to prevent this condition.
Figures



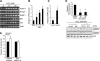
Similar articles
-
[Role of dysregulation of Bim in resistance of melanoma cells to endoplasmic reticulum stress-induced apoptosis].Zhonghua Zhong Liu Za Zhi. 2011 Jul;33(7):494-8. Zhonghua Zhong Liu Za Zhi. 2011. PMID: 22093624 Chinese.
-
FoxO proteins' nuclear retention and BH3-only protein Bim induction evoke mitochondrial dysfunction-mediated apoptosis in berberine-treated HepG2 cells.Free Radic Biol Med. 2014 Nov;76:185-99. doi: 10.1016/j.freeradbiomed.2014.07.039. Epub 2014 Aug 13. Free Radic Biol Med. 2014. PMID: 25128467
-
Aminophylline Improves Ventilator-Induced Diaphragmatic Dysfunction by Targeting IGF-1-FOXO1-MURF1 Pathway.Discov Med. 2024 Apr;36(183):699-713. doi: 10.24976/Discov.Med.202436183.66. Discov Med. 2024. PMID: 38665019
-
Ventilator-induced diaphragm dysfunction in critical illness.Exp Biol Med (Maywood). 2018 Dec;243(17-18):1329-1337. doi: 10.1177/1535370218811950. Epub 2018 Nov 19. Exp Biol Med (Maywood). 2018. PMID: 30453774 Free PMC article. Review.
-
Mechanical ventilation, diaphragm weakness and weaning: a rehabilitation perspective.Respir Physiol Neurobiol. 2013 Nov 1;189(2):377-83. doi: 10.1016/j.resp.2013.05.012. Epub 2013 May 18. Respir Physiol Neurobiol. 2013. PMID: 23692928 Free PMC article. Review.
Cited by
-
From bedside to recovery: exercise therapy for prevention of post-intensive care syndrome.J Intensive Care. 2024 Feb 29;12(1):11. doi: 10.1186/s40560-024-00724-4. J Intensive Care. 2024. PMID: 38424645 Free PMC article. Review.
-
Smad3 initiates oxidative stress and proteolysis that underlies diaphragm dysfunction during mechanical ventilation.Sci Rep. 2017 Nov 6;7(1):14530. doi: 10.1038/s41598-017-11978-4. Sci Rep. 2017. PMID: 29109401 Free PMC article.
-
Preferent Diaphragmatic Involvement in TK2 Deficiency: An Autopsy Case Study.Int J Mol Sci. 2021 May 25;22(11):5598. doi: 10.3390/ijms22115598. Int J Mol Sci. 2021. PMID: 34070501 Free PMC article.
-
Gene expression profile in the diaphragm following contractile inactivity during thoracic surgery.Int J Physiol Pathophysiol Pharmacol. 2011 Sep 30;3(3):167-75. Epub 2011 Sep 7. Int J Physiol Pathophysiol Pharmacol. 2011. PMID: 21941608 Free PMC article.
-
Rationale and design of a mechanistic clinical trial of JAK inhibition to prevent ventilator-induced diaphragm dysfunction.Respir Med. 2021 Nov-Dec;189:106620. doi: 10.1016/j.rmed.2021.106620. Epub 2021 Sep 21. Respir Med. 2021. PMID: 34655959 Free PMC article.
References
-
- Gayan-Ramirez G., Decramer M. (2002) Effects of mechanical ventilation on diaphragm function and biology. Eur. Respir. J. 20, 1579–1586 - PubMed
-
- Laghi F., Cattapan S., Jubran A., Parthasarathy S., Warshawsky P., Choi Y., Tobin M. (2003) Is weaning failure caused by low-frequency fatigue of the diaphragm? Am. J. Respir. Crit. Care Med. 167, 120–127 - PubMed
-
- Swartz M., Marino P. (1985) Diaphragmatic strength during weaning from mechanical ventilation. Chest 88, 736–739 - PubMed
-
- Esteban A., Frutos F., Tobin M., Alía I., Solsona J., Valverdú I., Fernández R., de la Cal M., Benito S., Tomás R. (1995) A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N. Engl. J. Med. 332, 345–350 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

