Cellular studies reveal mechanistic differences between taccalonolide A and paclitaxel
- PMID: 21597323
- PMCID: PMC3154365
- DOI: 10.4161/cc.10.13.16238
Cellular studies reveal mechanistic differences between taccalonolide A and paclitaxel
Abstract
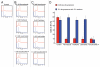
Taccalonolide A is a microtubule stabilizer that has cellular effects almost identical to paclitaxel. However, biochemical studies show that, unlike paclitaxel, taccalonolide A does not enhance purified tubulin polymerization or bind tubulin/microtubules. Mechanistic studies aimed at understanding the nature of the differences between taccalonolide A and paclitaxel were conducted. Our results show that taccalonolide A causes bundling of interphase microtubules at concentrations that cause antiproliferative effects. In contrast, the concentration of paclitaxel that initiates microtubule bundling is 31-fold higher than its IC 50. Taccalonolide A's effects are further differentiated from paclitaxel in that it is unable to enhance the polymerization of tubulin in cellular extracts. This finding extends previous biochemical results with purified brain tubulin to demonstrate that taccalonolide A requires more than tubulin and a full complement of cytosolic proteins to cause microtubule stabilization. Reversibility studies were conducted and show that the cellular effects of taccalonolide A persist after drug washout. In contrast, other microtubule stabilizers, including paclitaxel and laulimalide, demonstrate a much higher degree of cellular reversibility in both short-term proliferation and long-term clonogenic assays. The propensity of taccalonolide A to alter interphase microtubules at antiproliferative concentrations as well as its high degree of cellular persistence may explain why taccalonolide A is more potent in vivo than would be expected from cellular studies. The close linkage between the microtubule bundling and antiproliferative effects of taccalonolide A is of interest given the recent hypothesis that the effects of microtubule targeting agents on interphase microtubules might play a prominent role in their clinical anticancer efficacy.
Figures



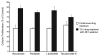
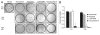
Comment in
-
Taccalonolides: a microtubule stabilizer poses a new puzzle with old pieces.Cell Cycle. 2011 Oct 1;10(19):3233-4. doi: 10.4161/cc.10.19.17126. Epub 2011 Oct 1. Cell Cycle. 2011. PMID: 21946521 Free PMC article. No abstract available.
References
-
- Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18(Suppl. 5):v3–v8. - PubMed
-
- Pusztai L. Markers predicting clinical benefit in breast cancer from microtubule-targeting agents. Ann Oncol. 2007;18(Suppl. 12):xii15–xii20. - PubMed
-
- Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Cabazitaxel. Nat Rev Drug Discov. 2010;9:677–678. - PubMed
-
- Morris PG, Fornier MN. Microtubule active agents: beyond the taxane frontier. Clin Cancer Res. 2008;14:7167–7172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
