Sub-lytic C5b-9 induces functional changes in retinal pigment epithelial cells consistent with age-related macular degeneration
- PMID: 21597483
- PMCID: PMC3178225
- DOI: 10.1038/eye.2011.109
Sub-lytic C5b-9 induces functional changes in retinal pigment epithelial cells consistent with age-related macular degeneration
Abstract
Purpose: There is evidence for complement dysfunction in age-related macular degeneration (AMD). Complement activation leads to formation of the membrane attack complex (MAC), known to assemble on retinal pigment epithelial (RPE) cells. Therefore, the effect of sub-lytic MAC on RPE cells was examined with regard to pro-inflammatory or pro-angiogenic mediators relevant in AMD.
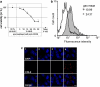
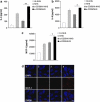
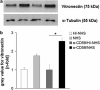
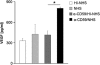
Methods: For sub-lytic MAC induction, RPE cells were incubated with an antiserum to complement regulatory protein CD59, followed by normal human serum (NHS) to induce 5% cell death, measured by a viability assay. MAC formation was evaluated by immunofluorescence and FACS analysis. Interleukin (IL)-6, -8, monocytic chemoattractant protein-1 (MCP-1), and vascular endothelial growth factor (VEGF) were quantified by enzyme-linked immunosorbent assay (ELISA). Intracellular MCP-1 was analysed by immunofluorescence, vitronectin by western blotting, and gelatinolytic matrix metalloproteinases (MMPs) by zymography.
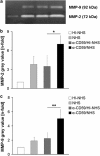
Results: Incubation of RPE cells with the CD59 antiserum followed by 5% NHS induced sub-lytic amounts of MAC, verified by FACS and immunofluorescence. This treatment stimulated the cells to release IL-6, -8, MCP-1, and VEGF. MCP-1 staining, production of vitronectin, and gelatinolytic MMPs were also elevated in response to sub-lytic MAC.
Conclusions: MAC assembly on RPE cells increases the IL-6, -8, and MCP-1 production. Therefore, sub-lytic MAC might have a significant role in generating a pro-inflammatory microenvironment, contributing to the development of AMD. Enhanced vitronectin might be a protective mechanism against MAC deposition. In addition, the increased expression of gelatinolytic MMPs and pro-angiogenic VEGF may be associated with neovascular processes and late AMD.
Figures
Similar articles
-
Complement-mediated release of fibroblast growth factor 2 from human RPE cells.Exp Eye Res. 2021 Mar;204:108471. doi: 10.1016/j.exer.2021.108471. Epub 2021 Jan 28. Exp Eye Res. 2021. PMID: 33516764
-
Complement activation by RPE cells preexposed to TNFα and IFNγ.Exp Eye Res. 2022 May;218:108982. doi: 10.1016/j.exer.2022.108982. Epub 2022 Feb 17. Exp Eye Res. 2022. PMID: 35183540
-
Complement Stimulates Retinal Pigment Epithelial Cells to Undergo Pro-Inflammatory Changes.Ophthalmic Res. 2015;54(4):195-203. doi: 10.1159/000439596. Epub 2015 Oct 27. Ophthalmic Res. 2015. PMID: 26502094
-
Should I stay or should I go? Trafficking of sub-lytic MAC in the retinal pigment epithelium.Adv Exp Med Biol. 2014;801:267-74. doi: 10.1007/978-1-4614-3209-8_34. Adv Exp Med Biol. 2014. PMID: 24664707 Review.
-
The role of complement membrane attack complex in dry and wet AMD - From hypothesis to clinical trials.Exp Eye Res. 2019 Jul;184:266-277. doi: 10.1016/j.exer.2019.05.006. Epub 2019 May 10. Exp Eye Res. 2019. PMID: 31082363 Review.
Cited by
-
Age-related increases in amyloid beta and membrane attack complex: evidence of inflammasome activation in the rodent eye.J Neuroinflammation. 2015 Jun 24;12:121. doi: 10.1186/s12974-015-0337-1. J Neuroinflammation. 2015. PMID: 26104676 Free PMC article.
-
C3aR1-Deletion Delays Retinal Degeneration in a White-Light Damage Mouse Model.Invest Ophthalmol Vis Sci. 2025 Jan 2;66(1):15. doi: 10.1167/iovs.66.1.15. Invest Ophthalmol Vis Sci. 2025. PMID: 39775695 Free PMC article.
-
Extracellular vesicles released by human retinal pigment epithelium mediate increased polarised secretion of drusen proteins in response to AMD stressors.J Extracell Vesicles. 2021 Nov;10(13):e12165. doi: 10.1002/jev2.12165. J Extracell Vesicles. 2021. PMID: 34750957 Free PMC article.
-
Modulation of three key innate immune pathways for the most common retinal degenerative diseases.EMBO Mol Med. 2018 Oct;10(10):e8259. doi: 10.15252/emmm.201708259. EMBO Mol Med. 2018. PMID: 30224384 Free PMC article. Review.
-
Action of the Terminal Complement Pathway on Cell Membranes.J Membr Biol. 2025 Aug;258(4):269-304. doi: 10.1007/s00232-025-00343-6. Epub 2025 Mar 23. J Membr Biol. 2025. PMID: 40122920 Free PMC article. Review.
References
-
- Skerka C, Lauer N, Weinberger AA, Keilhauer CN, Sühnel J, Smith R, et al. Defective complement control of factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol Immunol. 2007;44 (13:3398–3406. - PubMed
-
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. - PubMed
-
- Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58 (3:353–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

