Biologic markers determine both the risk and the timing of recurrence in breast cancer
- PMID: 21597921
- PMCID: PMC4324750
- DOI: 10.1007/s10549-011-1564-5
Biologic markers determine both the risk and the timing of recurrence in breast cancer
Abstract
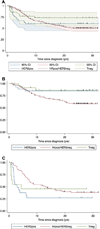
Breast cancer has a long natural history. Established and emerging biologic markers address overall risk but not necessarily timing of recurrence. 346 adjuvant naïve breast cancer cases from Guy's Hospital with 23 years minimum follow-up and archival blocks were recut and reassessed for hormone-receptors (HR), HER2-receptor and grade. Disease-specific survival (DSS) was analyzed by recursive partitioning. To validate insights from this analysis, gene-signatures (proliferative and HR-negative) were evaluated for their ability to predict early versus late metastatic risk in 683 node-negative, adjuvant naïve breast cancers annotated with expression microarray data. Risk partitioning showed that adjuvant naïve node-negative outcome risk was primarily partitioned by tumor receptor status and grade but not tumor size. HR-positive and HER2-negative (HRpos) risk was partitioned by tumor grade; low grade cases have very low early risk but a 20% fall-off in DSS 10 or more years after diagnosis. Higher grade HRpos cases have risk over >20 years. Triple-negative (Tneg) and HER2-positive (HER2pos) cases DSS events occurred primarily within the first 5 years. Among node-positive cases, only low grade conferred late risk, suggesting that proliferative gene signatures that identify proliferation would be important for predicting early but not late recurrence. Using pooled data from four publicly available data sets for node-negative tumors annotated with gene expression and outcome data, we evaluated four prognostic gene signatures: two proliferation-based and two immune function-based. Tumor proliferative capacity predicted early but not late metastatic risk for HRpos cases. The immune function or HRneg specific signatures predicted only early metastatic risk in Tneg and HER2pos cases. Breast cancer prognostic signatures need to inform both risk and timing of metastatic events and may best be applied within subsets. Current signatures predict for outcome risk within 5 years of diagnosis. Predictors of late risk for HR positive disease are needed.
Conflict of interest statement
Figures



References
-
- van‘t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002 Jan 31;415(6871):530–536. - PubMed
-
- Ross JS, Hatzis C, Symmans WF, Pusztai L, Hortobagyi GN. Commercialized multigene predictors of clinical outcome for breast cancer. Oncologist. 2008;13(5):477–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

