Hippocampal pyramidal cells: the reemergence of cortical lamination
- PMID: 21597968
- PMCID: PMC3197924
- DOI: 10.1007/s00429-011-0322-0
Hippocampal pyramidal cells: the reemergence of cortical lamination
Abstract
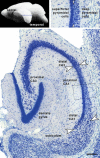
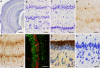
The increasing resolution of tract-tracing studies has led to the definition of segments along the transverse axis of the hippocampal pyramidal cell layer, which may represent functionally defined elements. This review will summarize evidence for a morphological and functional differentiation of pyramidal cells along the radial (deep to superficial) axis of the cell layer. In many species, deep and superficial sublayers can be identified histologically throughout large parts of the septotemporal extent of the hippocampus. Neurons in these sublayers are generated during different periods of development. During development, deep and superficial cells express genes (Sox5, SatB2) that also specify the phenotypes of superficial and deep cells in the neocortex. Deep and superficial cells differ neurochemically (e.g. calbindin and zinc) and in their adult gene expression patterns. These markers also distinguish sublayers in the septal hippocampus, where they are not readily apparent histologically in rat or mouse. Deep and superficial pyramidal cells differ in septal, striatal, and neocortical efferent connections. Distributions of deep and superficial pyramidal cell dendrites and studies in reeler or sparsely GFP-expressing mice indicate that this also applies to afferent pathways. Histological, neurochemical, and connective differences between deep and superficial neurons may correlate with (patho-) physiological phenomena specific to pyramidal cells at different radial locations. We feel that an appreciation of radial subdivisions in the pyramidal cell layer reminiscent of lamination in other cortical areas may be critical in the interpretation of studies of hippocampal anatomy and function.
© The Author(s) 2011. This article is published with open access at Springerlink.com
Figures




References
-
- Abbie AA. The relations of the fascia dentata, hippocampus and neocortex, and the nature of the subiculum. J Comp Neurol. 1938;68:307–333. doi: 10.1002/cne.900680303. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

