Docking glycosaminoglycans to proteins: analysis of solvent inclusion
- PMID: 21597992
- PMCID: PMC3107433
- DOI: 10.1007/s10822-011-9433-1
Docking glycosaminoglycans to proteins: analysis of solvent inclusion
Abstract
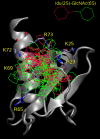
Glycosaminoglycans (GAGs) are anionic polysaccharides, which participate in key processes in the extracellular matrix by interactions with protein targets. Due to their charged nature, accurate consideration of electrostatic and water-mediated interactions is indispensable for understanding GAGs binding properties. However, solvent is often overlooked in molecular recognition studies. Here we analyze the abundance of solvent in GAG-protein interfaces and investigate the challenges of adding explicit solvent in GAG-protein docking experiments. We observe PDB GAG-protein interfaces being significantly more hydrated than protein-protein interfaces. Furthermore, by applying molecular dynamics approaches we estimate that about half of GAG-protein interactions are water-mediated. With a dataset of eleven GAG-protein complexes we analyze how solvent inclusion affects Autodock 3, eHiTs, MOE and FlexX docking. We develop an approach to de novo place explicit solvent into the binding site prior to docking, which uses the GRID program to predict positions of waters and to locate possible areas of solvent displacement upon ligand binding. To investigate how solvent placement affects docking performance, we compare these results with those obtained by taking into account information about the solvent position in the crystal structure. In general, we observe that inclusion of solvent improves the results obtained with these methods. Our data show that Autodock 3 performs best, though it experiences difficulties to quantitatively reproduce experimental data on specificity of heparin/heparan sulfate disaccharides binding to IL-8. Our work highlights the current challenges of introducing solvent in protein-GAGs recognition studies, which is crucial for exploiting the full potential of these molecules for rational engineering.
Figures

References
-
- Jackson R, Busch S, Cardin A. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Phys Rev. 1991;71:481–539. - PubMed
-
- Angulo J, Ojeda R, de Paz J, Lucas R, Nieto P, Lozano R, Redondo-Horcajo M, Giménez-Gallego G, Martín-Lomas M (2004) The activation of fibroblast growth factors (FGFs) by Glycosaminoglycans: influence of the sulfation pattern on the biological activity of FGF-1. Chem Bio Chem 5:55–61 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

