Tumor necrosis factor-α treatment of HepG2 cells mobilizes a cytoplasmic pool of ERp57/1,25D₃-MARRS to the nucleus
- PMID: 21598303
- PMCID: PMC3158835
- DOI: 10.1002/jcb.23187
Tumor necrosis factor-α treatment of HepG2 cells mobilizes a cytoplasmic pool of ERp57/1,25D₃-MARRS to the nucleus
Abstract
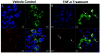
ERp57/PDIA3/1,25-MARRS has diverse functions and multiple cellular locations in various cell types. While classically described as an endoplasmic reticulum (ER) resident protein, ERp57 has a nuclear location sequence (NLS) and can enter the nucleus from the cytosol to alter transcription of target genes. Dysregulation and variable expression of ERp57 is associated with a variety of cancers including hepatocellular carcinoma (HCC). We investigated the dynamic mobility of ERp57 in an HCC cell line, HepG2, to better understand the movement and function of the non-ER resident pool of ERp57. Subcellular fractionation indicated ERp57 is highly expressed in the ER with a smaller cytoplasmic pool in HepG2 cells. Utilizing an ERp57 green fluorescent protein fusion construct created with and without a secretory signal sequence, we found that cytoplasmic ERp57 translocated to the nucleus within 15 min after tumor necrosis factor-α (TNF-α) treatment. Protein kinase C activators including 1,25-dihydroxyvitamin D(3) and phorbol myristate acetate did not trigger nuclear translocation of ERp57, indicating translocation is PKC independent. To determine if an interaction between the rel homology binding domain in ERp57 and the nuclear factor-κB subunit, p65, occurred after TNF-α treatment and could account for nuclear movement, co-immunoprecipitation was performed under control and conditions that stabilized labile disulfide bonds. No support for a functional interaction between p65 and ERp57 after TNF-α treatment was found in either case. Immunostaining for both ERp57-GFP and p65 after TNF-α treatment indicated that nuclear translocation of these two proteins occurs independently in HepG2 cells.
Copyright © 2011 Wiley-Liss, Inc.
Figures




References
-
- Bourdi M, Demady D, Martin JL, Jabbour SK, Martin BM, George JW, Pohl LR. cDNA cloning and baculovirus expression of the human liver endoplasmic reticulum P58: characterization as a protein disulfide isomerase isoform, but not as a protease or a carnitine acyltransferase. Arch Biochem Biophys. 1995;323:397–403. - PubMed
-
- Chichiarelli S, Ferraro A, Altieri F, Eufemi M, Coppari S, Grillo C, Arcangeli V, Turano C. The stress protein ERp57/GRP58 binds specific DNA sequences in HeLa cells. J Cell Physiol. 2007;210:343–51. - PubMed
-
- Chignard N, Shang S, Wang H, Marrero J, Brechot C, Hanash S, Beretta L. Cleavage of endoplasmic reticulum proteins in hepatocellular carcinoma: Detection of generated fragments in patient sera. Gastroenterology. 2006;130:2010–22. - PubMed
-
- Cicchillitti L, Della Corte A, Di Michele M, Donati MB, Rotilio D, Scambia G. Characterisation of a multimeric protein complex associated with ERp57 within the nucleus in paclitaxel-sensitive and -resistant epithelial ovarian cancer cells: the involvement of specific conformational states of beta-actin. Int J Oncol. 2010;37:445–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

