Apolipoprotein A-I mimetic peptide L-4F prevents myocardial and coronary dysfunction in diabetic mice
- PMID: 21598304
- PMCID: PMC3158830
- DOI: 10.1002/jcb.23188
Apolipoprotein A-I mimetic peptide L-4F prevents myocardial and coronary dysfunction in diabetic mice
Abstract
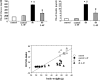
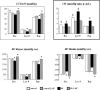
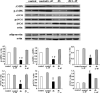
Diabetes is a major health problem associated with adverse cardiovascular outcomes. The apolipoprotein A-I mimetic peptide L-4F is a putative anti-diabetic drug, has antioxidant and anti-inflammatory proprieties and improves endothelial function. In obese mice L-4F increases adiponectin levels, improving insulin sensitivity, and reducing visceral adiposity. We hypothesized that the pleiotropic actions of L-4F can prevent heart and coronary dysfunction in a mouse model of genetically induced Type II diabetes. We treated db/db mice with either L-4F or vehicle for 8 weeks. Trans-thoracic echocardiography was performed; thereafter, isolated hearts were subjected to ischemia/reperfusion (IR). Glucose, insulin, adiponectin, and pro-inflammatory cytokines (IL-1β, TNF-α, MCP-1) were measured in plasma and HO-1, pAMPK, peNOS, iNOS, adiponectin, and superoxide in cardiac tissue. In db/db mice L-4F decreased accumulation of subcutaneous and total fat, and increased insulin sensitivity and adiponectin levels while lowering inflammatory cytokines (P < 0.05). L-4F normalized in vivo left ventricular (LV) function of db/db mice, increasing (P < 0.05) fractional shortening and decreasing (P < 0.05) LV dimensions. In I/R experiments, L-4F prevented coronary microvascular resistance from increasing and LV function from deteriorating in the db/db mice. These changes were associated with increased cardiac expression of HO-1, pAMPK, peNOS, and adiponectin and decreased levels of superoxide and iNOS (P < 0.01). In the present study we showed that L-4F prevented myocardial and coronary functional abnormalities in db/db mice. These effects were associated with stimulation of HO-1 resulting in increased levels of anti-inflammatory, anti-oxidative, and vasodilatatory action through a mechanism involving increased levels of adiponectin, pAMPK, and peNOS.
Copyright © 2011 Wiley-Liss, Inc.
Figures
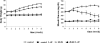
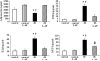


References
-
- Abraham NG, Kushida T, McClung J, Weiss M, Quan S, Lafaro R, Darzynkiewicz Z, Wolin M. Heme oxygenase-1 attenuates glucose-mediated cell growth arrest and apoptosis in human microvessel endothelial cells. Circ.Res. 2003;93:507–514. - PubMed
-
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. - PubMed
-
- Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. - PubMed
-
- Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295(5):E1269–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

