Carbon electrode fabrication from pyrolyzed parylene C
- PMID: 21599013
- PMCID: PMC3128194
- DOI: 10.1021/ac200885w
Carbon electrode fabrication from pyrolyzed parylene C
Abstract
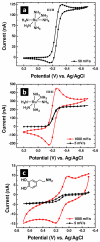
Carbon electrodes coupled with electrochemical detection have been used extensively for the investigation of biogenic amines. Herein we report the fabrication and characterization of carbonaceous electrodes prepared from pyrolyzed parylene C (PPC) films. High-aspect ratio carbonaceous microelectrodes have been prepared by masking PPC coated pipets with an insulating parylene C film. PPC thin film electrodes were characterized electrochemically, spectroscopically, and with electron microscopy. The procedures described here offer a route to fabrication of thin film carbon electrodes that can be patterned and produced in parallel. These electrodes are similar to carbon electrodes derived from pyrolyzed photoresist films but do not require spin-coating or lithography and can readily coat three-dimensional surfaces.
Figures

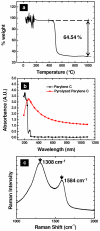
 ) deposited on a quartz substrate. (c) Averaged Raman spectra (n = 10) of amorphous pyrolyzed parylene. Characteristic G and D modes are visible at 1308 cm−1 and 1583 cm−1 respectively in (d). λexc = 785 nm, 10 mW power average.
) deposited on a quartz substrate. (c) Averaged Raman spectra (n = 10) of amorphous pyrolyzed parylene. Characteristic G and D modes are visible at 1308 cm−1 and 1583 cm−1 respectively in (d). λexc = 785 nm, 10 mW power average.

Similar articles
-
Pyrolyzed carbon film diodes.ACS Appl Mater Interfaces. 2013 Nov 13;5(21):10673-81. doi: 10.1021/am402758y. Epub 2013 Oct 24. ACS Appl Mater Interfaces. 2013. PMID: 24090451
-
Parylene insulated probes for scanning electrochemical-atomic force microscopy.Langmuir. 2011 Nov 15;27(22):13925-30. doi: 10.1021/la203032u. Epub 2011 Oct 24. Langmuir. 2011. PMID: 21961960
-
Carbon electrode obtained via pyrolysis of plasma-deposited parylene-C for electrochemical immunoassays.Analyst. 2022 Aug 8;147(16):3783-3794. doi: 10.1039/d2an00854h. Analyst. 2022. PMID: 35876175
-
Conformal coating using parylene polymers.Med Device Technol. 1997 Jan-Feb;8(1):14-20. Med Device Technol. 1997. PMID: 10167681 Review.
-
Molecular dynamics simulations of the growth of poly(chloro-para-xylylene) films.J Mol Model. 2011 Nov;17(11):2725-33. doi: 10.1007/s00894-011-1050-3. Epub 2011 May 6. J Mol Model. 2011. PMID: 21547549 Free PMC article. Review.
Cited by
-
Spectroscopic and electrochemical characterization of nanostructured optically transparent carbon electrodes.Electrophoresis. 2013 Jul;34(14):1998-2006. doi: 10.1002/elps.201300022. Epub 2013 Jun 21. Electrophoresis. 2013. PMID: 23595607 Free PMC article.
-
3D-Printed Carbon Electrodes for Neurotransmitter Detection.Angew Chem Int Ed Engl. 2018 Oct 22;57(43):14255-14259. doi: 10.1002/anie.201809992. Epub 2018 Oct 4. Angew Chem Int Ed Engl. 2018. PMID: 30207021 Free PMC article.
-
Fabrication, characterization, and functionalization of dual carbon electrodes as probes for scanning electrochemical microscopy (SECM).Anal Chem. 2013 Aug 6;85(15):7519-26. doi: 10.1021/ac401476z. Epub 2013 Jul 8. Anal Chem. 2013. PMID: 23795948 Free PMC article.
-
Development and Characterization of Carbon Based Electrodes from Pyrolyzed Paper for Biosensing Applications.J Electroanal Chem (Lausanne). 2016 Mar 15;765:8-15. doi: 10.1016/j.jelechem.2015.07.055. Epub 2015 Aug 16. J Electroanal Chem (Lausanne). 2016. PMID: 27175108 Free PMC article.
-
Array Microcell Method (AMCM) for Serial Electroanalysis.ChemElectroChem. 2020 Mar 2;7(5):1084-1091. doi: 10.1002/celc.201901976. Epub 2020 Jan 9. ChemElectroChem. 2020. PMID: 36588586 Free PMC article.
References
-
- Wightman RM, Strope E, Plotsky PM, Adams RN. Nature. 1976;262:145–146. - PubMed
-
- Strand AM, Venton BJ. Anal. Chem. 2008;80:3708–3715. - PubMed
-
- Blackstock JJ, Rostami AA, Nowak AM, McCreery RL, Freeman MR, McDermott MT. Anal. Chem. 2004;76:2544–2552. - PubMed
-
- Kawagoe KT, Jankowski JA, Wightman RM. Anal. Chem. 1991;63:1589–1594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

