A microstructurally driven model for pulmonary artery tissue
- PMID: 21599093
- PMCID: PMC3108498
- DOI: 10.1115/1.4002698
A microstructurally driven model for pulmonary artery tissue
Abstract
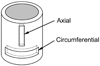
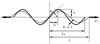
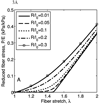
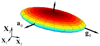
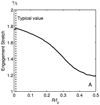
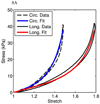
A new constitutive model for elastic, proximal pulmonary artery tissue is presented here, called the total crimped fiber model. This model is based on the material and microstructural properties of the two main, passive, load-bearing components of the artery wall, elastin, and collagen. Elastin matrix proteins are modeled with an orthotropic neo-Hookean material. High stretch behavior is governed by an orthotropic crimped fiber material modeled as a planar sinusoidal linear elastic beam, which represents collagen fiber deformations. Collagen-dependent artery orthotropy is defined by a structure tensor representing the effective orientation distribution of collagen fiber bundles. Therefore, every parameter of the total crimped fiber model is correlated with either a physiologic structure or geometry or is a mechanically measured material property of the composite tissue. Further, by incorporating elastin orthotropy, this model better represents the mechanics of arterial tissue deformation. These advancements result in a microstructural total crimped fiber model of pulmonary artery tissue mechanics, which demonstrates good quality of fit and flexibility for modeling varied mechanical behaviors encountered in disease states.
Figures













References
-
- Hunter KS, Lee PF, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, Chan KC, Shandas R. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. American Heart Journal. 2008;155(1):166–174. - PMC - PubMed
-
- Humphrey JD. Cardiovascular solid mechanics : cells, tissues, and organs. New York: Springer; 2002.
-
- Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R. Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am. J. Physiol.-Heart Circul. Physiol. 2008;295(4):H1451–H1459. - PMC - PubMed
-
- Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed. Pharmacother. 2003;57(5–6):195–202. - PubMed
-
- Patel DJ, Fry DL. Elastic Symmetry of Arterial Segments in Dogs. Circulation Research. 1969;24(1) pp. 1-&. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

