Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer
- PMID: 21599634
- PMCID: PMC3670669
- DOI: 10.2174/156800911796191024
Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer
Abstract
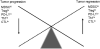
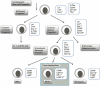
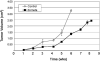
Recent studies describe a heterogeneous population of cells of the myeloid lineage, termed myeloid derived suppressor cells (MDSC), which are observed with increased prevalence in the peripheral blood and tumor microenvironment of cancer patients, including pancreatic cancer. Accumulation of MDSC in the peripheral circulation has been related to extent of disease, and correlates with stage. MDSC have primarily been implicated in promoting tumor growth by suppressing antitumor immunity. There is also compelling evidence MDSC are also involved in angiogenesis and metastatic spread. Two main subsets of MDSC have been identified in cancer patients: a monocytic subset, characterized by expression of CD14, and a granulocytic subset characterized by expression of CD15. Both subsets of MDSC actively suppress host immunity through a variety of mechanisms including production of reactive oxygen species and arginase. Just as in humans, accumulation of monocytic and granulocytic MDSC has been noted in the bone marrow, spleen, peripheral circulation, and tumors of tumor bearing mice. Successful targeting of MDSC in mice is associated with improved immune responses, delayed tumor growth, improved survival, and increased efficacy of vaccine therapy. By further elucidating mechanisms of MDSC recruitment and maintenance in the tumor environment, strategies could be developed to reverse immune tolerance to tumor. We discuss here what is currently known about MDSC as well as some potential strategies targeting MDSC in the context of our work on pancreatic cancer and recent literature. Due to the number of new reports on MDSC, the most pertinent ones have been selected.
Figures


References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. - PubMed
-
- Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control. 2006;17:403–409. - PubMed
-
- Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. J.O.P. 2008;9:99–132. - PubMed
-
- Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. 2006;10:1199–1210. - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–2413. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

