Specific treatment of problems of the spine (STOPS): design of a randomised controlled trial comparing specific physiotherapy versus advice for people with subacute low back disorders
- PMID: 21599941
- PMCID: PMC3121656
- DOI: 10.1186/1471-2474-12-104
Specific treatment of problems of the spine (STOPS): design of a randomised controlled trial comparing specific physiotherapy versus advice for people with subacute low back disorders
Abstract
Background: Low back disorders are a common and costly cause of pain and activity limitation in adults. Few treatment options have demonstrated clinically meaningful benefits apart from advice which is recommended in all international guidelines. Clinical heterogeneity of participants in clinical trials is hypothesised as reducing the likelihood of demonstrating treatment effects, and sampling of more homogenous subgroups is recommended. We propose five subgroups that allow the delivery of specific physiotherapy treatment targeting the pathoanatomical, neurophysiological and psychosocial components of low back disorders. The aim of this article is to describe the methodology of a randomised controlled trial comparing specific physiotherapy treatment to advice for people classified into five subacute low back disorder subgroups.
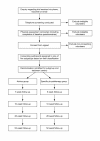
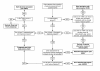
Methods/design: A multi-centre parallel group randomised controlled trial is proposed. A minimum of 250 participants with subacute (6 weeks to 6 months) low back pain and/or referred leg pain will be classified into one of five subgroups and then randomly allocated to receive either physiotherapy advice (2 sessions over 10 weeks) or specific physiotherapy treatment (10 sessions over 10 weeks) tailored according to the subgroup of the participant. Outcomes will be assessed at 5 weeks, 10 weeks, 6 months and 12 months following randomisation. Primary outcomes will be activity limitation measured with a modified Oswestry Disability Index as well as leg and back pain intensity measured on separate 0-10 Numerical Rating Scales. Secondary outcomes will include a 7-point global rating of change scale, satisfaction with physiotherapy treatment, satisfaction with treatment results, the Sciatica Frequency and Bothersomeness Scale, quality of life (EuroQol-5D), interference with work, and psychosocial risk factors (Orebro Musculoskeletal Pain Questionnaire). Adverse events and co-interventions will also be measured. Data will be analysed according to intention to treat principles, using linear mixed models for continuous outcomes, Mann Whitney U tests for ordinal outcomes, and Chi-square, risk ratios and risk differences for dichotomous outcomes.
Discussion: This trial will determine the difference in outcomes between specific physiotherapy treatment tailored to each of the five subgroups versus advice which is recommended in guidelines as a suitable treatment for most people with a low back disorder.
Trial registration: Australia and New Zealand Clinical Trials Register (ANZCTR): ACTRN12609000834257.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical