Comparisons of resistance of CF and non-CF pathogens to hydrogen peroxide and hypochlorous acid oxidants in vitro
- PMID: 21599970
- PMCID: PMC3118099
- DOI: 10.1186/1471-2180-11-112
Comparisons of resistance of CF and non-CF pathogens to hydrogen peroxide and hypochlorous acid oxidants in vitro
Abstract
Background: Cystic fibrosis (CF) lung disease has a unique profile of pathogens predominated by Pseudomonas aeruginosa (PsA) and Staphylococcus aureus (SA). These microorganisms must overcome host immune defense to colonize the CF lungs. Polymorphonuclear neutrophils are a major component of the host defense against bacterial infection. A crucial microbicidal mechanism is the production of oxidants including hydrogen peroxide (H2O2) and hypochlorous acid (HOCl) by neutrophils to achieve efficient bacterial killing. To determine to what degrees various CF pathogens resist the oxidants relative to non-CF pathogens, we compared the susceptibility of PsA, SA, Burkholderia cepacia (BC), Klebsiella pneumoniae (KP), and Escherichia coli (EC) to various concentrations of H2O2 or HOCl, in vitro. The comparative oxidant-resistant profiles were established. Oxidant-induced damage to ATP production and cell membrane integrity of the microbes were quantitatively assessed. Correlation of membrane permeability and ATP levels with bacterial viability was statistically evaluated.
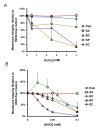
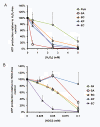
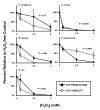
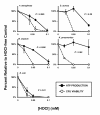
Results: PsA was relatively resistant to both H2O2 (LD50 = 1.5 mM) and HOCl (LD50 = 0.035 mM). SA was susceptible to H2O2 (LD50 = 0.1 mM) but resistant to HOCl (LD50 = 0.035 mM). Interestingly, KP was extremely resistant to high doses of H2O2 (LD50 = 2.5-5.0 mM) but was very sensitive to low doses of HOCl (LD50 = 0.015 mM). BC was intermediate to resist both oxidants: H2O2 (LD50 = 0.3-0.4 mM) and HOCl (LD50 = 0.025 mM). EC displayed the least resistance to H2O2 (LD50 = 0.2-0.3 mM) and HOCl (LD50 = 0.015 mM). The identified profile of H2O2-resistance was KP > PsA > BC > EC > SA and the profile of HOCl-resistance PsA > SA > BC > EC > KP. Moreover, both oxidants affected ATP production and membrane integrity of the cells. However, the effects varied among the tested organisms and, the oxidant-mediated damage correlated differentially with the bacterial viability.
Conclusions: The order of HOCl-resistance identified herein best fits the clinical profile of CF infections. Even though oxidants are able to disrupt ATP production and cell membrane integrity, the degrees of damage vary among the organisms and correlate differentially with their viability.
Figures



References
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting G. In: Metabolic and Molecular Basis of Interited Disease. 8. Scriver CR, editor. New York: McGraw-Hill; 2001. Cystic Fibrosis; pp. 5121–5188.
-
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5):1229–1256. - PubMed
-
- Foundation CF. Cystic Fibrosis Foundation Patient Rigestry: 2009 Annual Data Report. http://www.cff.org/UploadedFiles/research/ClinicalResearch/Patient-Regis...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

