Therapeutic potential of N-acetylcysteine as an antiplatelet agent in patients with type-2 diabetes
- PMID: 21600014
- PMCID: PMC3120650
- DOI: 10.1186/1475-2840-10-43
Therapeutic potential of N-acetylcysteine as an antiplatelet agent in patients with type-2 diabetes
Abstract
Background: Platelet hyperaggregability is a pro-thrombotic feature of type-2 diabetes, associated with low levels of the antioxidant glutathione (GSH). Clinical delivery of N-acetylcysteine (NAC), a biosynthetic precursor of GSH, may help redress a GSH shortfall in platelets, thereby reducing thrombotic risk in type-2 diabetes patients. We investigated the effect of NAC in vitro, at concentrations attainable with tolerable oral dosing, on platelet GSH concentrations and aggregation propensity in blood from patients with type-2 diabetes.
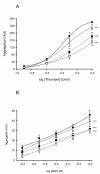
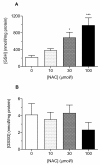
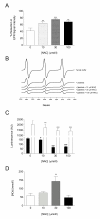
Methods: Blood samples (n = 13) were incubated (2 h, 37°C) with NAC (10-100 micromolar) in vitro. Platelet aggregation in response to thrombin and ADP (whole blood aggregometry) was assessed, together with platelet GSH concentration (reduced and oxidized), antioxidant status, reactive oxygen species (ROS) generation, and plasma NOx (a surrogate measure of platelet-derived nitric oxide; NO).
Results: At therapeutically relevant concentrations (10-100 micromolar), NAC increased intraplatelet GSH levels, enhanced the antioxidant effects of platelets, and reduced ROS generation in blood from type-2 diabetes patients. Critically, NAC inhibited thrombin- and ADP-induced platelet aggregation in vitro. Plasma NOx was enhanced by 30 micromolar NAC.
Conclusions: Our results suggest that NAC reduces thrombotic propensity in type-2 diabetes patients by increasing platelet antioxidant status as a result of elevated GSH synthesis, thereby lowering platelet-derived ROS. This may increase bioavailability of protective NO in a narrow therapeutic range. Therefore, NAC might represent an alternative or additional therapy to aspirin that could reduce thrombotic risk in type-2 diabetes.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

