Dissection of the factors driving the placebo effect in hypnotic treatment of depressed insomniacs
- PMID: 21601519
- PMCID: PMC3110560
- DOI: 10.1016/j.sleep.2011.03.008
Dissection of the factors driving the placebo effect in hypnotic treatment of depressed insomniacs
Abstract
Objectives: Our prior work has shown that there is improvement in self-reported sleep in persons receiving placebo in hypnotic clinical trials. We examined the components of the "placebo response" in a hypnotic clinical trial.
Methods: This was an exploratory analysis of a randomized, double-blind clinical trial of eszopiclone versus placebo in the treatment of persons with depression and insomnia who were also receiving fluoxetine at a clinic of a teaching hospital. Sixty adults with both depression and insomnia symptoms, who were free of significant primary sleep disorders, received open-label fluoxetine for 9weeks. Patients were further randomized 1:1 to receive either masked eszopiclone 3mg or placebo at bedtime after the first week of fluoxetine. We examined the respective contributions of three factors associated with the "placebo effect": (1) regression to the mean, (2) expectancy, and (3) social desirability.
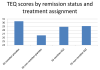
Results: There was evidence for regression to the mean for the continuous measurement of the Insomnia Severity Index (ISI) and the Hamilton Depression Rating Scale. There was evidence for expectancy in self-reported Wake After Sleep Onset, continuous measurement of ISI, and dichotomous remission/non-remitter measurement of ISI. There was evidence of social desirability affecting self-reported Total Sleep Time.
Conclusions: Factors that have been associated with the "placebo effect" are operating in hypnotic clinical trials. However, the role of each factor differs depending upon which self-reported variable is being considered. The findings have implications for clinical trial design in insomnia.
Trial registration: ClinicalTrials.gov NCT00247624.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures




References
-
- Miller F, Rosenstein DL. Varience and Dissent: The Nature and Power of the Placebo Effect. J Clin Exp Neuropsychol. 2006;59:331–335. - PubMed
-
- Beecher HK. Surgery as Placebo. A Quantitative Study of Bias. JAMA. 1961;176:1102–1107. - PubMed
-
- McCall V, Perlis M, Tu X, et al. A comparison of placebo and no-treatment during a hypnotic clinical trial. Int J Clin Pharm Ther. 2005;43:355–359. - PubMed
-
- DiMasi J. New drug development in the United States from 1963 to 1999. Clin Pharmacol Ther. 2001;69:286–296. - PubMed
-
- DiMasi J. Risks in new drug development: Approval success rates for investigational drugs. Clin Pharmacol Ther. 2001;69:297–307. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

