Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide
- PMID: 21602333
- PMCID: PMC3147541
- DOI: 10.1128/JB.00311-11
Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide
Abstract
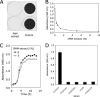
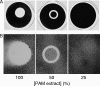
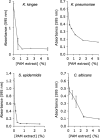
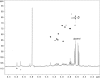
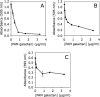
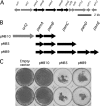
Cell-free extracts prepared from Kingella kingae colony biofilms were found to inhibit biofilm formation by Aggregatibacter actinomycetemcomitans, Klebsiella pneumoniae, Staphylococcus aureus, Staphylococcus epidermidis, Candida albicans, and K. kingae. The extracts evidently inhibited biofilm formation by modifying the physicochemical properties of the cell surface, the biofilm matrix, and the substrate. Chemical and biochemical analyses indicated that the biofilm inhibition activity in the K. kingae extract was due to polysaccharide. Structural analyses showed that the extract contained two major polysaccharides. One was a linear polysaccharide with the structure →6)-α-d-GlcNAcp-(1→5)-β-d-OclAp-(2→, which was identical to a capsular polysaccharide produced by Actinobacillus pleuropneumoniae serotype 5. The second was a novel linear polysaccharide, designated PAM galactan, with the structure →3)-β-d-Galf-(1→6)-β-d-Galf-(1→. Purified PAM galactan exhibited broad-spectrum biofilm inhibition activity. A cluster of three K. kingae genes encoding UDP-galactopyranose mutase (ugm) and two putative galactofuranosyl transferases was sufficient for the synthesis of PAM galactan in Escherichia coli. PAM galactan is one of a growing number of bacterial polysaccharides that exhibit antibiofilm activity. The biological roles and potential technological applications of these molecules remain unknown.
Figures

References
-
- Altman E., Brisson J.-R., Perry M. B. 1987. Structure of the capsular polysaccharide of Haemophilus pleuropneumoniae serotype 5. Eur. J. Biochem. 170:185–192 - PubMed
-
- Berbari E. F., Cockerill F. R., Stickelberg J. M. 1997. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin. Proc. 72:532–542 - PubMed
-
- Bock K., Pedersen C. 1983. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. Adv. Carbohydr. Chem. Biochem. 41:27–66
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

