Responses of Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus to simulated food processing treatments, determined using fluorescence-activated cell sorting and plate counting
- PMID: 21602370
- PMCID: PMC3127720
- DOI: 10.1128/AEM.00323-11
Responses of Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus to simulated food processing treatments, determined using fluorescence-activated cell sorting and plate counting
Abstract
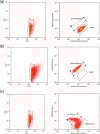
Three common food pathogenic microorganisms were exposed to treatments simulating those used in food processing. Treated cell suspensions were then analyzed for reduction in growth by plate counting. Flow cytometry (FCM) and fluorescence-activated cell sorting (FACS) were carried out on treated cells stained for membrane integrity (Syto 9/propidium iodide) or the presence of membrane potential [DiOC2(3)]. For each microbial species, representative cells from various subpopulations detected by FCM were sorted onto selective and nonselective agar and evaluated for growth and recovery rates. In general, treatments giving rise to the highest reductions in counts also had the greatest effects on cell membrane integrity and membrane potential. Overall, treatments that impacted cell membrane permeability did not necessarily have a comparable effect on membrane potential. In addition, some bacterial species with extensively damaged membranes, as detected by FCM, appeared to be able to replicate and grow after sorting. Growth of sorted cells from various subpopulations was not always reflected in plate counts, and in some cases the staining protocol may have rendered cells unculturable. Optimized FCM protocols generated a greater insight into the extent of the heterogeneous bacterial population responses to food control measures than did plate counts. This study underlined the requirement to use FACS to relate various cytometric profiles generated by various staining protocols with the ability of cells to grow on microbial agar plates. Such information is a prerequisite for more-widespread adoption of FCM as a routine microbiological analytical technique.
Figures

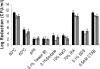


References
-
- Ananta E., Knorr D. 2009. Comparison of inactivation pathways of thermal or high pressure inactivated Lactobacillus rhamnosus ATCC 53103 by flow cytometry analysis. Food Microbiol. 26:542–546 - PubMed
-
- Brul S., Coote P. 1999. Preservative agents in foods: mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 50:1–17 - PubMed
-
- Campos F. M., et al. 2009. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 135:144–151 - PubMed
-
- Coder D. M. 2001. Assessment of cell viability. John Wiley & Sons, Inc., Hoboken, NJ.
-
- Cronin U. P., Wilkinson M. G. 2008. Bacillus cereus endospores exhibit a heterogeneous response to heat treatment and low-temperature storage. Food Microbiol. 25:235–243 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

