Transcatheter intraarterial therapies: rationale and overview
- PMID: 21602502
- PMCID: PMC3400295
- DOI: 10.1148/radiol.11081489
Transcatheter intraarterial therapies: rationale and overview
Abstract
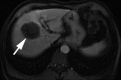
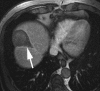
Transcatheter intraarterial therapies have proved valuable in the battle against primary and secondary hepatic malignancies. The unique aspects of all such therapies are their reduced toxicity profiles and highly effective tumor responses. These unique characteristics coupled with their minimally invasive nature provide an attractive therapeutic option in patients who may have previously had few alternatives. The concept of all catheter-based intraarterial therapies is to selectively deliver anticancer treatment to tumor(s). These therapies, which include transarterial embolization, intraarterial chemoinfusion, transarterial chemoembolization with or without drug-eluting beads, and radioembolization with use of yttrium 90, inflict lethal insult to tumors while preserving normal hepatic parenchyma. This is possible because hepatic neoplasms preferentially derive their blood supply from an arterial source while the majority of noncancerous liver is supplied by the portal vein. As part of the interventional oncology review series, in this article we describe the rationale behind each of these transcatheter therapies and provide a review of the existing medical literature.
RSNA, 2011
Figures























References
-
- Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46(3):474–481 - PubMed
-
- Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006;29(4):522–529 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

