Antagonism of nerve growth factor-TrkA signaling and the relief of pain
- PMID: 21602663
- PMCID: PMC3121917
- DOI: 10.1097/ALN.0b013e31821b1ac5
Antagonism of nerve growth factor-TrkA signaling and the relief of pain
Abstract
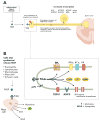
Nerve growth factor (NGF) was originally discovered as a neurotrophic factor essential for the survival of sensory and sympathetic neurons during development. However, in the adult NGF has been found to play an important role in nociceptor sensitization after tissue injury. The authors outline mechanisms by which NGF activation of its cognate receptor, tropomyosin-related kinase A receptor, regulates a host of ion channels, receptors, and signaling molecules to enhance acute and chronic pain. The authors also document that peripherally restricted antagonism of NGF-tropomyosin-related kinase A receptor signaling is effective for controlling human pain while appearing to maintain normal nociceptor function. Understanding whether there are any unexpected adverse events and how humans may change their behavior and use of the injured/degenerating tissue after significant pain relief without sedation will be required to fully appreciate the patient populations that may benefit from these therapies targeting NGF.
Conflict of interest statement
Figures



References
-
- Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803–12. - PubMed
-
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. - PubMed
-
- Fishman SM, Teichera D. Challenges and choices in drug therapy for chronic pain. Cleve Clin J Med. 2003;70:119–38. - PubMed
-
- Katz WA, Barkin RL. Dilemmas in chronic/persistent pain management. Dis Mon. 2010;56:233–50. - PubMed
-
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

