Clinical management and follow-up of hypercholesterolemia among perinatally HIV-infected children enrolled in the PACTG 219C study
- PMID: 21602698
- PMCID: PMC3159838
- DOI: 10.1097/QAI.0b013e31822203f5
Clinical management and follow-up of hypercholesterolemia among perinatally HIV-infected children enrolled in the PACTG 219C study
Abstract
Background: Hypercholesterolemia is common in perinatally HIV-infected (HIV+) children, but little is known about the clinical course and management in this population.
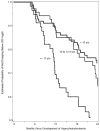
Methods: We studied HIV+ children in a multisite prospective cohort study (Pediatric AIDS Clinical Trials Group 219C) and considered follow-up for 2 years after development of hypercholesterolemia. We estimated the time and factors associated with resolution of hypercholesterolemia and described changes in antiretroviral regimen and use of lipid-lowering medications. We defined incident hypercholesterolemia as entry total cholesterol (cholesterol) <220 mg/dL and 2 subsequent consecutive cholesterol ≥ 220 mg/dL and defined resolution of hypercholesterolemia as 2 consecutive cholesterol <200 mg/dL after incident hypercholesterolemia.
Results: Among 240 incident hypercholesterolemia cases, 81 (34%) had resolution to normal cholesterol within 2 years of follow-up (median follow-up = 1.9 years). The median age of cases was 10.3 years with 54% non-Hispanic black and 53% male. Resolution to normal cholesterol was more likely in children who changed antiretroviral regimen (adjusted hazard ratio = 2.37, 95% confidence interval: 1.45 to 3.88) and who were 13 years and older (aHR = 2.39, 95% confidence interval: 1.33 to 4.27). Types of regimen changes varied greatly, and 15 children began statins.
Conclusion: The majority of children who develop hypercholesterolemia maintain elevated levels over time, potentially placing them at risk for premature cardiovascular morbidity.
Conflict of interest statement
The authors have no conflict of interest.
Figures
Comment in
-
Cardiovascular disease risk in pediatric HIV: the need for population-specific guidelines.J Acquir Immune Defic Syndr. 2011 Aug 15;57(5):351-4. doi: 10.1097/QAI.0b013e318227b016. J Acquir Immune Defic Syndr. 2011. PMID: 21694605 No abstract available.
References
-
- Grunfeld C, Pang M, Doerrler W, et al. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–52. - PubMed
-
- EL-Sadr WM, Mullin CM, Carr A, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Medicine. 2005;6:114–21. - PubMed
-
- Constans J, Pellegrin JL, Peuchant E, et al. Plasma lipids in HIV-infected patients: a prospective study in 95 patients. Eur J Clin Invest. 1994;24:416–20. - PubMed
-
- Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. J Am Med Assoc. 2003;289:2978–82. - PubMed
-
- Grunfeld C, Kotler DPSJK, Doerrler W, et al. Circulating interfereon-alpha levels and hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1991;90:154–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical