Subcellular spatial regulation of canonical Wnt signalling at the primary cilium
- PMID: 21602792
- PMCID: PMC3107376
- DOI: 10.1038/ncb2259
Subcellular spatial regulation of canonical Wnt signalling at the primary cilium
Abstract
Mechanisms of signal transduction regulation remain a fundamental question in a variety of biological processes and diseases. Previous evidence indicates that the primary cilium can act as a signalling hub, but its exact role in many of the described pathways has remained elusive. Here, we investigate the mechanism of cilia-mediated regulation of the canonical Wnt pathway. We found that primary cilia dampen canonical Wnt signalling through a spatial mechanism involving compartmentalization of signalling components. The cilium, through regulated intraflagellar transport, diverts Jouberin (Jbn), a ciliopathy protein and context-specific Wnt pathway regulator, away from the nucleus and limits β-catenin nuclear entry. This repressive regulation does not silence the pathway, but instead maintains a discrete range of Wnt responsiveness; cells without cilia have potentiated Wnt responses, whereas cells with multiple cilia have inhibited responses. Furthermore, we show that this regulation occurs during embryonic development and is disrupted in cancer cell proliferation. Together these data explain a spatial mechanism of Wnt signalling regulation that may provide insight into ciliary regulation of other signalling pathways.
Figures
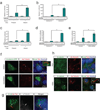
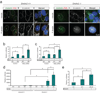

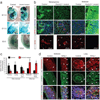
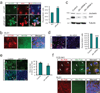
Comment in
-
Wnt signalling escapes to cilia.Nat Cell Biol. 2011 Jun;13(6):636-7. doi: 10.1038/ncb0611-636. Nat Cell Biol. 2011. PMID: 21633348
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

