A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells
- PMID: 21602800
- PMCID: PMC3681612
- DOI: 10.1038/nm.2375
A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells
Abstract
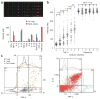
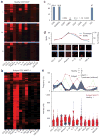
Cellular immunity has an inherent high level of functional heterogeneity. Capturing the full spectrum of these functions requires analysis of large numbers of effector molecules from single cells. We report a microfluidic platform designed for highly multiplexed (more than ten proteins), reliable, sample-efficient (∼1 × 10(4) cells) and quantitative measurements of secreted proteins from single cells. We validated the platform by assessment of multiple inflammatory cytokines from lipopolysaccharide (LPS)-stimulated human macrophages and comparison to standard immunotechnologies. We applied the platform toward the ex vivo quantification of T cell polyfunctional diversity via the simultaneous measurement of a dozen effector molecules secreted from tumor antigen-specific cytotoxic T lymphocytes (CTLs) that were actively responding to tumor and compared against a cohort of healthy donor controls. We observed profound, yet focused, functional heterogeneity in active tumor antigen-specific CTLs, with the major functional phenotypes quantitatively identified. The platform represents a new and informative tool for immune monitoring and clinical assessment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. - PubMed
-
- O’Shea JJ, Hunter CA, Germain RN. T cell heterogeneity: firmly fixed, predominantly plastic or merely malleable? Nat Immunol. 2008;9:450–453. - PubMed
-
- Darrah PA, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. - PubMed
-
- Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

