Lrp5 functions in bone to regulate bone mass
- PMID: 21602802
- PMCID: PMC3113461
- DOI: 10.1038/nm.2388
Lrp5 functions in bone to regulate bone mass
Abstract
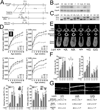
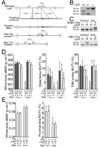
The human skeleton is affected by mutations in low-density lipoprotein receptor-related protein 5 (LRP5). To understand how LRP5 influences bone properties, we generated mice with osteocyte-specific expression of inducible Lrp5 mutations that cause high and low bone mass phenotypes in humans. We found that bone properties in these mice were comparable to bone properties in mice with inherited mutations. We also induced an Lrp5 mutation in cells that form the appendicular skeleton but not in cells that form the axial skeleton; we observed that bone properties were altered in the limb but not in the spine. These data indicate that Lrp5 signaling functions locally, and they suggest that increasing LRP5 signaling in mature bone cells may be a strategy for treating human disorders associated with low bone mass, such as osteoporosis.
Figures




Comment in
-
The holy grail of high bone mass.Nat Med. 2011 Jun;17(6):657-8. doi: 10.1038/nm0611-657. Nat Med. 2011. PMID: 21647140 No abstract available.
-
Bone: Evidence for local effects of LRP5 on bone mass.Nat Rev Rheumatol. 2011 Jun 21;7(7):373. doi: 10.1038/nrrheum.2011.84. Nat Rev Rheumatol. 2011. PMID: 21691327 No abstract available.
-
Lrp5 regulation of bone mass and serotonin synthesis in the gut.Nat Med. 2014 Nov;20(11):1228-9. doi: 10.1038/nm.3698. Nat Med. 2014. PMID: 25375916 No abstract available.
-
Reply to Lrp5 regulation of bone mass and gut serotonin synthesis.Nat Med. 2014 Nov;20(11):1229-30. doi: 10.1038/nm.3697. Nat Med. 2014. PMID: 25375917 Free PMC article. No abstract available.
References
-
- Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. - PubMed
-
- Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. - PubMed
-
- Balemans W, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

