The SK2-long isoform directs synaptic localization and function of SK2-containing channels
- PMID: 21602822
- PMCID: PMC3417338
- DOI: 10.1038/nn.2832
The SK2-long isoform directs synaptic localization and function of SK2-containing channels
Abstract
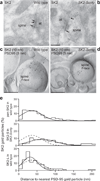
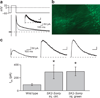
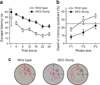
SK2-containing channels are expressed in the postsynaptic density (PSD) of dendritic spines on mouse hippocampal area CA1 pyramidal neurons and influence synaptic responses, plasticity and learning. The Sk2 gene (also known as Kcnn2) encodes two isoforms that differ only in the length of their N-terminal domains. SK2-long (SK2-L) and SK2-short (SK2-S) are coexpressed in CA1 pyramidal neurons and likely form heteromeric channels. In mice lacking SK2-L (SK2-S only mice), SK2-S-containing channels were expressed in the extrasynaptic membrane, but were excluded from the PSD. The SK channel contribution to excitatory postsynaptic potentials was absent in SK2-S only mice and was restored by SK2-L re-expression. Blocking SK channels increased the amount of long-term potentiation induced in area CA1 in slices from wild-type mice but had no effect in slices from SK2-S only mice. Furthermore, SK2-S only mice outperformed wild-type mice in the novel object recognition task. These results indicate that SK2-L directs synaptic SK2-containing channel expression and is important for normal synaptic signaling, plasticity and learning.
Figures




References
-
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. - PubMed
-
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. - PubMed
-
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. - PubMed
-
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. - PubMed
-
- Harris AZ, Pettit DL. Recruiting extrasynaptic NMDA receptors augments synaptic signaling. J Neurophysiol. 2008;99:524–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

